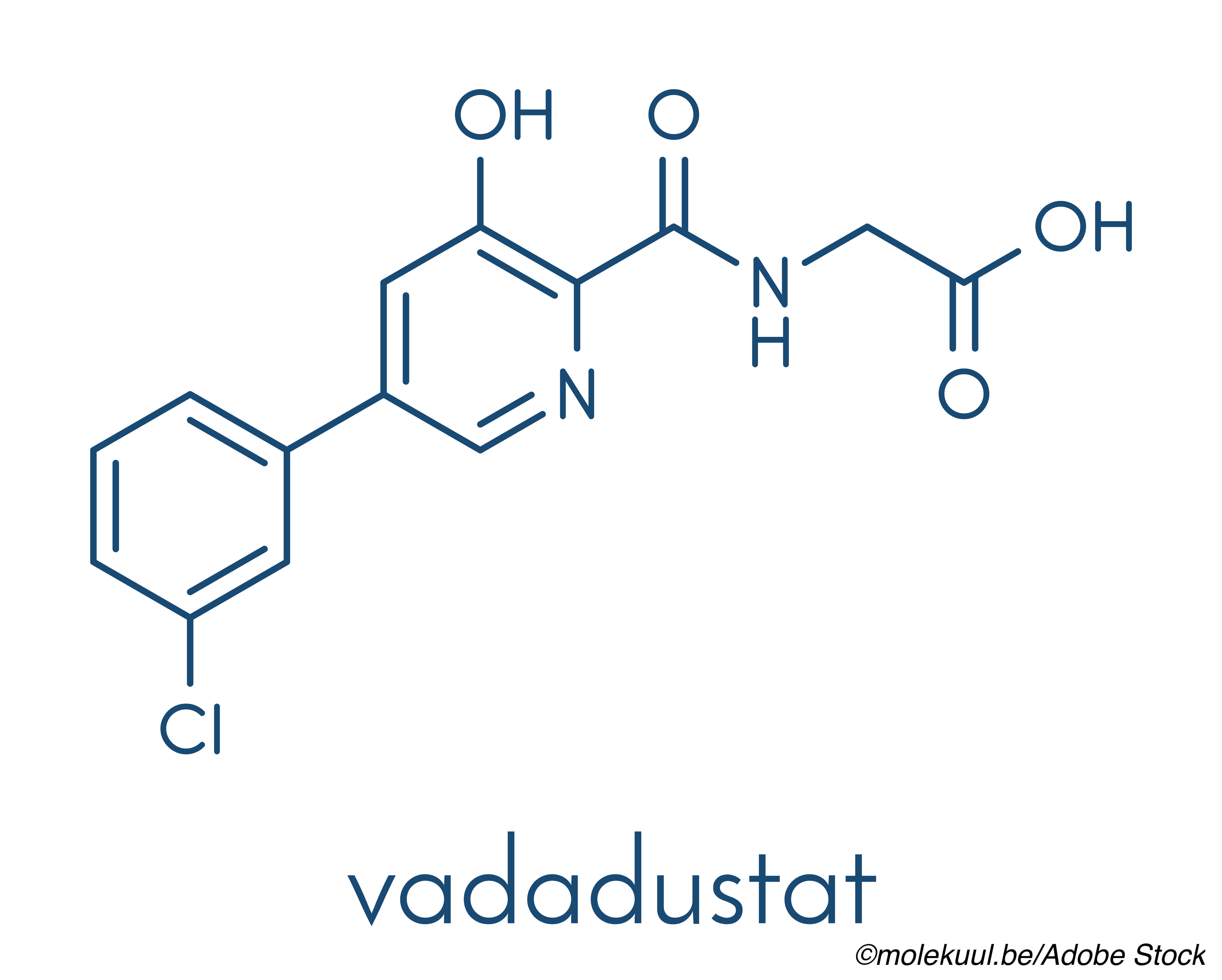
Results from the full clinical trial program comparing the investigational drug vadadustat, an oral hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor, to darbepoetin alfa, a commonly used parental erythropoiesis-stimulating agent (ESA) for the treatment of anemia in dialysis and non-dialysis dependent (NDD)-chronic kidney disease (CKD), showed noninferiority for vadadustat for both safety and efficacy, the primary difference being in the safety endpoint of major adverse cardiovascular events (MACE) in NDD-CKD patients, where MACE rates were higher for vadadustat than rates for the ESA arm.
In two phase III, randomized controlled trials in dialysis-dependent (DD)-CKD, investigators compared the noninferiority of vadadustat with that of darbepoetin alfa in patients with anemia and either incident or prevalent dialysis-dependent CKD. In the pooled analysis of both trials, a first MACE occurred in 18.2% of the vadadustat group compared with 19.3% in the darbepoetin alfa group (Hazard Ratio [HR], 0.96, 95% CI, 0.83-1.11), Kai-Uwe Eckhardt, MD, Charité–Universitätsmedizin, Berlin, Germany, and colleagues from the INNO2VATE study group reported in The New England Journal of Medicine.
In the overall clinical trial program, MACE was defined as death from any cause, nonfatal myocardial infarction, or nonfatal stroke.
In the incident DD-CKD trial, the mean change in hemoglobin from baseline to average values during weeks 24 to 36 was 1.26 g/dL in the vadadustat group and 1.58 g/dL in the ESA-treatment group. Corresponding changes in hemoglobin from baseline to average values during weeks 40 to 52 were again similar between the two groups at 1.42 g/dL for vadadustat and 1.50 g/dL for darbepoetin alfa, the investigators added.
In the prevalent DD-CKD trial, the mean change in hemoglobin from baseline to average values during weeks 24 to 36 was numerically lower at 0.19 g/dL in the non-ESA arm compared to 0.36 g/dL in the ESA arm, with corresponding changes from baseline to average values during weeks 40 to 52 again being at least numerically lower in the non-ESA arm at 0.23 g/dL versus 0.41 g/dL for the ESA arm.
The same two drugs were also compared in two other phase III, noninferiority trials in the non-dialysis dependent-CKD population, where patients were stratified into those who had not been previously treated with an ESA and those who were on active ESA-maintenance therapy from Glenn Chertow, MD, MPH, Stanford University School of Medicine, Palo Alto, California and colleagues from the PRO2TECT study group and reported in the same issue of The New England Journal of Medicine.
This pooled analysis of both PRO2TECT trials showed that a first MACE occurred in 22% of the vadadustat group compared with 19.9% in the darbepoetin alfa group (HR, 1.17; 95% CI, 1.01-1.36), which did not meet the prespecified noninferiority margin of 1.25.
In the NDD-CKD trial involving ESA-untreated patients, mean changes in hemoglobin from baseline to mean values at weeks 24 through 36 were 1.43 g/dL in the vadadustat group and 1.38 g/dL in the darbepoetin alfa group, with corresponding mean changes at week 40 through 52 of 1.52 g/dL in the non-ESA arm versus 1.48 g/dL in the ESA arm.
Similarly, mean changes in hemoglobin from baseline to mean values at weeks 24 through to 36 in the PRO2TECT trial involving ESA-treated patients were virtually identical in both groups at 0.41 g/dL in the vadadustat arm and 0.42 g/dL in the darbepoetin alfa arm, which was also the case at weeks 40 through 52, where mean changes in the same efficacy endpoint were 0.43 g/dL and 0.44 g/dL in each group, respectively.
“Given the concern about harms with ESAs, the discovery and development of a new class of agents to treat anemia—hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors—is exciting,” editorialist Adeera Levin, MD, University of British Columbia, Vancouver, BC, observed in an accompanying commentary on the clinical trials program.
“The data are convincing that vadadustat is effective in increasing hemoglobin concentrations in both dialysis-dependent and non-dialysis-dependent populations but are less convincing with respect to safety,” she said, adding that: “The issues raised in these trials should motivate us to answer critical questions regarding goals of therapy, risks, and benefits, with trials specifically designed to do so,” Levin advised.
Both INNO2VATE studies enrolled a total of 3,923 patients on dialysis who had a serum ferritin concentration of at least 100 ng/mL and a transferrin saturation of at least 20%.
“In addition, patients had a hemoglobin concentration between 8 and 11 g per deciliter (incident DD-CKD trial) or a hemoglobin concentration between 8 and 11 g per deciliter (patients in the United States) or between 9 and 12 g per deciliter (patients in other countries) [in the] prevalent DD-CKD trial,” the investigators wrote.
The PRO2TECT trials in turn enrolled patients with an estimated glomerular filtration rate (eGFR) of 60 mL/min/1.73 m2 or less.
ESA-untreated patients in PRO2TECT were required to have a hemoglobin concentration of less than 10 g/dL on study entry while ESA-treated patients had to be actively receiving maintenance ESA.
The same levels of serum ferritin and transferring saturation were required for study entry into PRO2TECT as were required for entry into the INNO2VATE studies.
A total of 1,751 patients with ESA-untreated non-dialysis dependent-CKD and 1,725 patients on maintenance ESA non-dialysis dependent-CKD were randomized into the 2 trials.
In all 4 studies, the starting dose of vadadustat was 300 mg, taken by mouth once a day, with other doses being available for dose adjustment up to a maximum of 600 mg a day.
The initial dose of darbepoetin alfa, in turn, was based on the previous dose or on information contained on the product label in case patients had not been previously treated with the drug. Doses were adjusted to achieve a target hemoglobin of 10 to 11 g/dL in the US or 10 to 12 g/dL in other countries, and the use of iron supplementation in whatever formulation was encouraged to maintain baseline serum ferritin and transferring saturation required for entry criteria.
The cumulative pooled probability of a first expanded MACE—a secondary safety end point in the overall clinical program—in the INNO2VATE trials was 21.6% in the vadadustat group and 23% in the ESA group (HR, 0.96; 95% CI, 0.84-1.10).
In the NDD-CKD PRO2TECT study, the probability of a first expanded MACE occurred in 25.9% of the non-ESA group compared with 24.5% in the ESA group (HR, 1.11; 95% CI, 0.97-1.27), as Chertow and colleagues noted.
In all 4 studies, expanded MACE included MACE plus hospitalization for heart failure or a thromboembolic event plus the original components of MACE.
The number of patients who achieved a hemoglobin concentration within their country-specific target range in the INNO2VATE trials was 43.6% in the vadadustat arms compared with 56.9% in the darbepoetin alfa arms at weeks 24 to 36 and 39.8% and 41%, respectively, during weeks 40 to 52. Across the clinical trials program, rates of serious drug-related adverse events were low and comparable in both treatment groups.
“The difference in outcomes between the current pooled trials involving patients with NDD-CKD and the pooled trials involving patients with DD-CKD is not the first example in which the relative safety of drugs used to treat anemia differed between dialysis dependent-CKD and non-dialysis dependent-CKD populations,” Chertow and colleagues pointed out.
For example, in the Peginesatide for the Correction of Anemia in Patients with Chronic Renal Failure Not on Dialysis and Not Receiving Treatment with Erythropoiesis-Stimulating Agents, or PEARL, trials, peginesatide did not meet its noninferiority margin for safety compared with darbepoetin alfa, as the PRO2TECT authors noted.
In the current PRO2TECT trials, analysis of the safety end points showed that the higher risk of MACE observed among patients assigned to the vadadustat arm was largely due to an excess of nonfatal myocardial infarctions and a higher incidence of death from noncardiovascular causes, although the reason for this discrepancy remains unexplained, the authors stressed.
-
Vadadustat, a non-ESA agent, was shown to be noninferior to a commonly used ESA agent for the treatment of anemia in dialysis and non-dialysis dependent CKD in terms of its effect on hemoglobin across 4 randomized controlled trials.
-
At least in the setting of non-dialysis dependent CKD, the new oral agent was associated with a higher rate of MACE compared to the ESA agent for still undetermined reasons.
Pam Harrison, Contributing Writer, BreakingMED™
The PRO2TECt and INNO2VATE trials were funded by Akebia Therapeutics and Osaka Pharmaceutical.
Chertow reported receiving advisory board fees and owning stock options in Ardelyx, Miromatrix Medical, and Unicycive Therapeutics, receiving steering committee fees from AstraZeneca, Gilead Sciences, Sanifit, and Vertex Pharmaceuticals, receiving advisory board fees from Baxter, Cricket Health, DiaMedica Therapeutics, and Reata Pharmaceuticals, serving on an advisory board and owning stock options in CloudCath, Durect, and Outset Medical, receiving fees for serving on a data and safety monitoring board from Angion, Bayer, and ReCor Medical, and receiving grant support, paid to his institution, from Amgen.
Eckardt reported consultant and or grant contracts with Akebia Therapeutics, Amgen Inc., Bayer Healthcare and Vifor Pharma.
Levin reported grants and other from AstraZeneca, outside the submitted work.
Cat ID: 446
Topic ID: 74,446,730,446,914,12,187,127,472,192,669,925