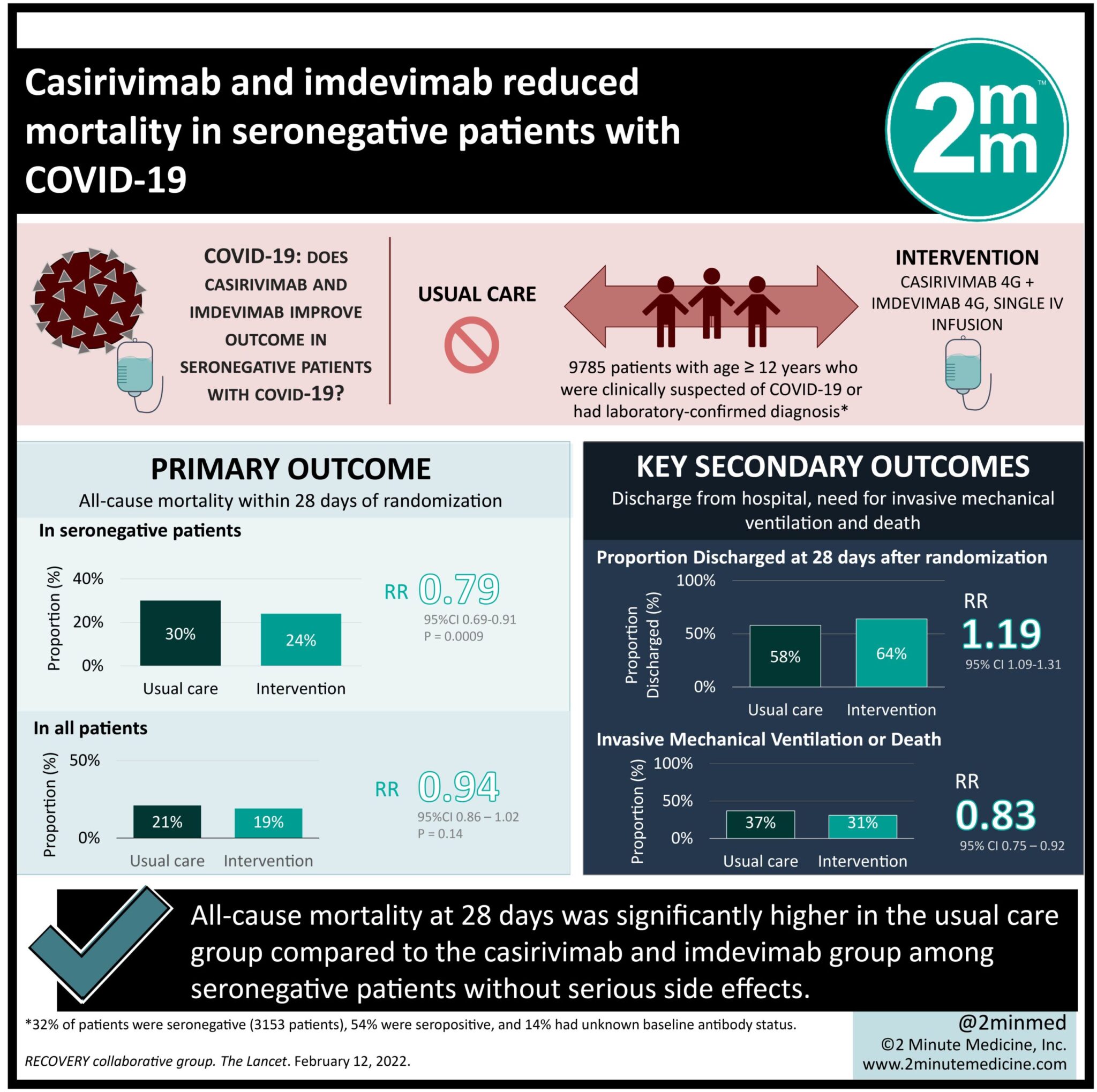
1. All-cause mortality at 28 days was significantly higher in the usual care group compared to the casirivimab and imdevimab group among seronegative patients.
2. Proportion of serious adverse events were comparable between groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Monoclonal antibodies are agents that may target a specific receptor on the host cell. Casirivimab and imdevimab are two non-competing, high-affinity IgG1 SARS-CoV-2 antibodies that may inhibit viral entry into host cells by targeting the spike glycoprotein. This randomized controlled trial aimed to compare the safety and efficacy of casirivimab and imdevimab versus usual care alone in patients with clinically suspected or laboratory-confirmed COVID-19. The primary outcome was all-cause mortality within 28 days of randomization while key secondary outcomes included time to discharge from hospital, need for invasive mechanical ventilation and death. According to study results, there 28-day mortality was significant reduced in the intervention group, compared to control for patients known to be seronegative at baseline. This reduction in mortality was not seen in the overall population. Furthermore, the number of serious adverse events in either group was comparable with no treatment related deaths. This study was limited in generalizability by a relatively old age group who is known to be at higher risk of, and worsened, COVID-19. Stratifying individuals by age and comparing the efficacy of treatments on each group may further validate the study findings.
Click to read the study in The Lancet
Relevant Reading: Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19
In-depth [randomized-controlled trial]: Between Sept 18, 2020, and May 22, 2021, 24 343 patients were assessed for eligibility across 127 UK hospitals. Included were those ≥12 years of age with clinically suspected or laboratory-confirmed COVID-19. Altogether, 9785 patients (4839 in the experimental plus usual care group and 4946 in usual care group) were included in the final analysis. Mean age for enrolled patients was 61.9 years (standard deviation [SD] 14.5). The primary outcome of all-cause mortality within 28 days of randomization was significantly higher in the usual care group (n=452, 30%) compared to the intervention group for seronegative patients (n=396, 24%, rate ratio [RR] 0.79, 95% confidence interval [CI] 0.69-0.91, p=0.0009). Secondary outcomes of time to hospital discharge (13 days vs. 17 days), need for mechanical ventilation (12% vs. 14%, RR 0.87), and death (24% vs. 29%, RR 0.82) favored casirivimab and imdevimab over usual care in the seronegative groups. Conversely, there was no mortality benefit seen in the overall population when including patients with seropositivity or unknown status at baseline. The effect of casirivimab and imdevimab on mortality was significantly different between patients who were seronegative and seropositive at baseline (test for heterogeneity p=0.002). Overall, findings from this study suggest that a combination of casirivimab and imdevimab was superior to placebo in reducing all-cause mortality at 28-days in patients who were seronegative for COVID-19.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.