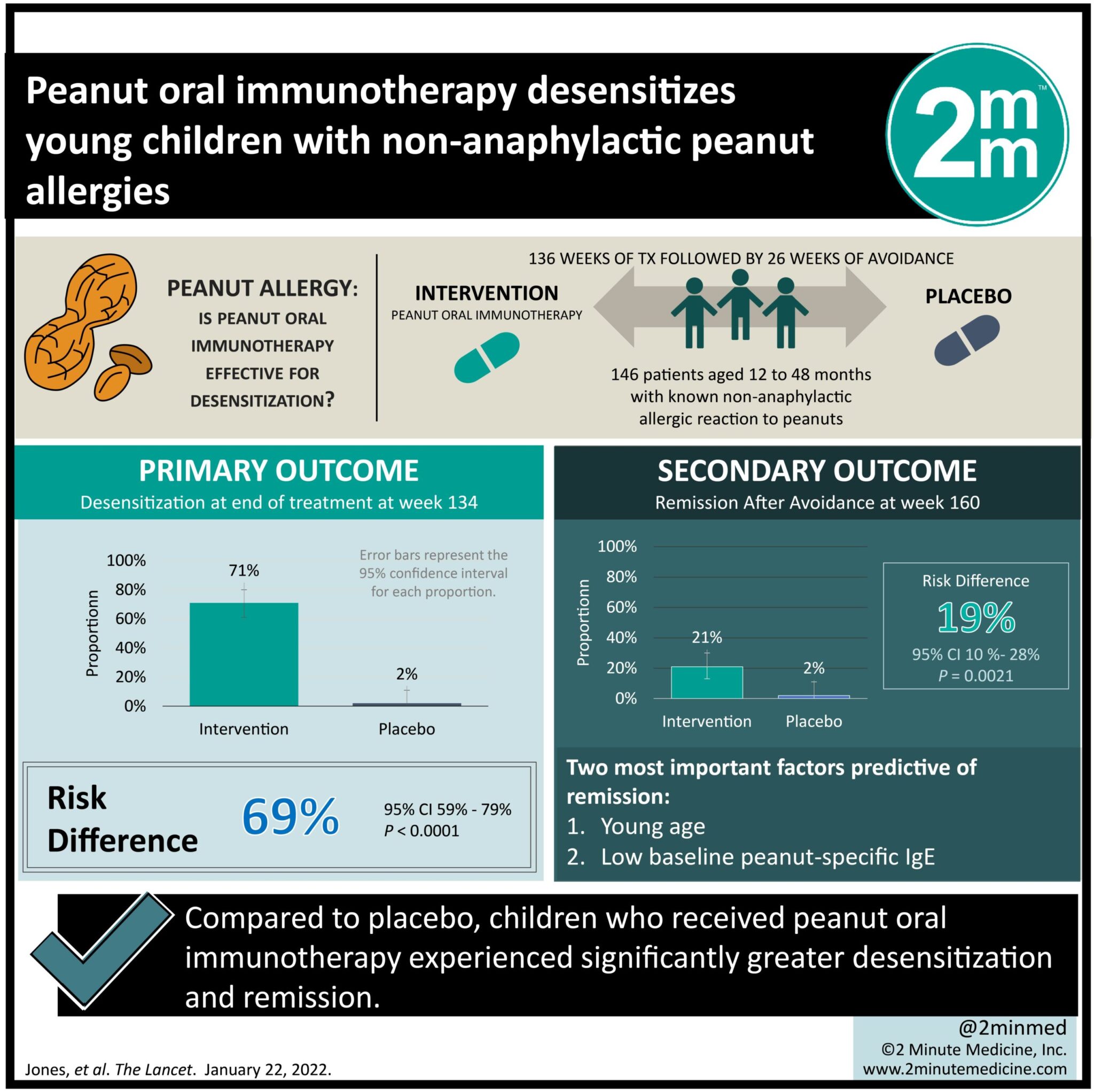
- Compared to placebo, children who received peanut oral immunotherapy experienced significantly greater desensitization and remission.
- Younger age at randomization was a positive predictive factor for remission.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Peanut allergy remains a major dietary concern in the United States. Studies show that most children with a peanut allergy may never grow out of it. The current standard of care for these children is dietary avoidance. However, the immature immune system of young children may represent a time where interventions may be more effective. This randomized controlled trial aimed to assess whether peanut oral immunotherapy could effectively induce desensitization and remission in children with non-anaphylactic peanut allergy. The primary outcome was post-therapeutic desensitization at week 134, while key secondary outcome was remission at week 160. According to study results, a significantly greater proportion of children in the peanut oral immunotherapy group achieved desensitization and remission compared with placebo. A major limitation of this study is that it only included children born in the United States. Since environmental allergies, specifically to peanuts, vary according to geographical origin, it may be interesting to compare the results of this study to children born in other countries.
Click to read the study in The Lancet
Relevant Reading: AR101 Oral Immunotherapy for Peanut Allergy
In-depth [randomized-controlled trial]: Between Aug 13, 2013, and Oct 1, 2015, 209 patients were assessed for eligibility across five US academic medical centers. Included were those 12 to 48 months of age with known non-anaphylactic allergic reaction to peanuts. Altogether, 146 children (96 in oral immunotherapy group and 50 in placebo group) were included in the final analysis. The primary outcome of desensitization at week 134 (end of treatment) was significantly greater in the peanut oral immunotherapy group (71%, 95% confidence interval [CI] 61-80) compared with placebo (2%, 95% CI 0.05-11, risk difference [RD] 69%, p<0.0001). Likewise, more children achieved remission at week 160 (after a 26-week avoidance period) while on oral immunotherapy (21%, 95% CI 13-30) than placebo (2%, 95% CI 0.05-11), although the risk difference was lower (19%, p=0.0021). The two most important factors predictive of remission were young age and low baseline peanut specific IgE. Majority of adverse events were mild to moderate in nature and managed using epinephrine. Overall, findings from this study suggest that administration of peanut oral immunotherapy may increase desensitization and remission among children with peanut allergy.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.