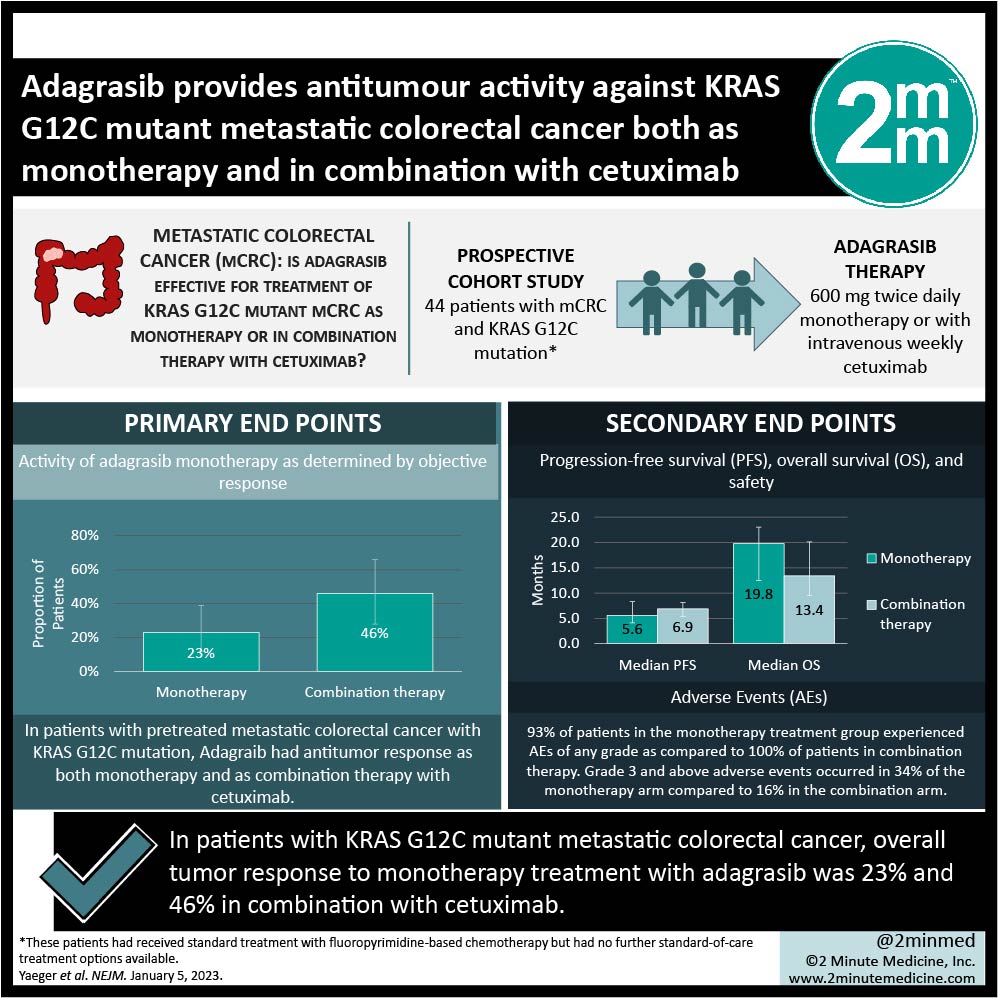
- Overall tumour response to monotherapy treatment with adagrasib was 23% 46% in combination with cetuximab.
- Median progression-free survival was 5.6 months on adagrasib monotherapy and 6.9 months on combination therapy with cetuximab.
Evidence Rating Level: 2 (Good)
Study Rundown: Kirsten rat sarcoma virus oncogene homologue (KRAS) is responsible for oncogenesis in almost half of the patients diagnosed with colorectal cancer (CRC). The prognosis is worse for patients with the KRAS G12C allele. The small-molecule adagrasib is a covalent inhibitor of KRAS G12C and renders it inactive. This phase 1-2 clinical trial investigated the use of adagrasib as a single-agent treatment for metastatic CRC (mCRC) with mutated KRAS G12C as well as in combination with cetuximab in heavily pre-treated patients. The primary outcome of this study was the activity of adagrasib monotherapy as determined by objective response. Secondary outcomes include progression-free survival (PFS), overall survival (OS), and the safety of the study drug. The objective response to adagrasib was 23% as compared to 46% of combination therapy patients. The median PFS was 5.6 months in the single therapy adagrasib group, whereas it was 6.9 months in the combination group. Median OS was 19.8 in the adagrasib group and 13.4 months in the combination treatment group. Adverse events (AEs) occurred in the majority of patients including 93% in the adagrasib group and 100% in the combination group. Diarrhea was a common AE between the groups, as was nausea and vomiting. Limitations to this study include that it was a non-randomized trial, which prevents directly comparing the two treatment groups and that the study enrolled small numbers of patients into each group, limiting the generalizability of the results. Overall, the results from this study provide evidence that adagrasib monotherapy and its combination with cetuximab as a treatment for pretreated mCRC with KRAS G12C mutation is a potentially useful therapy requiring further investigation.
Click to read the study in The New England Journal of Medicine
Relevant Reading: KRAS G12C metastatic colorectal cancer: specific features of a new emerging target population.
In-Depth [prospective cohort]: This open-label trial was non-randomized and recruited adult patients with mCRC and KRAS G12C mutation who had received standard treatment with fluoropyrimidine-based chemotherapy, but had no further standard-of-care treatment options available at enrollment. Forty-four patients with mCRC were provided with adagrasib monotherapy, while 32 patients received a combination of adagrasib and cetuximab. Of the monotherapy group, only 43 patients were clinically evaluable, whereas only 28 of the combination group were. The activity of adagrasib was determined by the overall response to the drug. According to a blinded central review, 23% of those receiving monotherapy had a response (95% confidence interval (CI), 12-39%). The objective response to the combination therapy was 46% of the 28 patients (95% CI, 28-66%). Medium PFS in the monotherapy group was 5.6 months (95% CI, 4.1 – 8.3 months) and median OS was 19.8 months (95% CI, 12.5 – 23.0). Median PFS among all 32 combination therapy patients was 6.9 months (95% CI, 5.4 – 8.1 months) and the median OS was 13.4 months (95% CI, 9.5-20.1 months). 93% of patients in the monotherapy treatment group experienced AEs of any grade as compared to 100% of patients in combination therapy. Grade 3 and above adverse events occurred in 34% of the monotherapy arm compared to 16% in the combination arm.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.