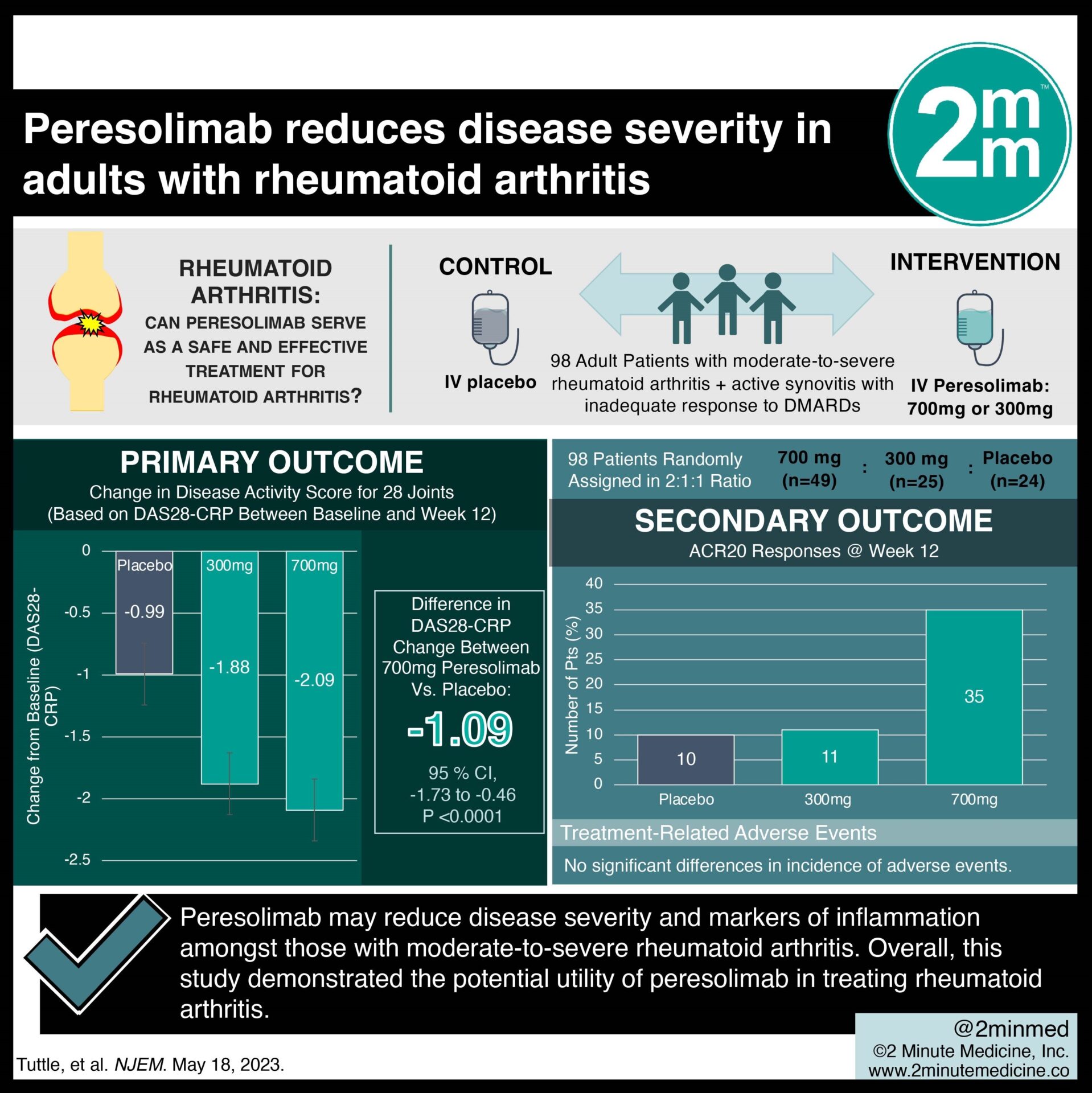
1. In this randomized controlled trial, there was a significant reduction in the Disease Activity Score of rheumatoid arthritis patients taking peresolimab compared to a placebo.
2. Peresolimab had a comparable adverse event profile to the placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Rheumatoid arthritis is a systemic, autoimmune inflammatory disease for which remission and sustained remission are challenging. Disease-modifying antirheumatic drugs (DMARDs) have been a cornerstone in treating rheumatoid arthritis. However, their response often diminishes over time for a significant portion of patients. Peresolimab, a monoclonal IgG1 antibody, is a novel therapeutic in the potential treatment of rheumatoid arthritis, which exerts its effect by stimulating the cell death protein (PD-1) inhibitory pathway. This randomized placebo-controlled study of 98 patients evaluated the efficacy and safety profile of peresolimab in adults with moderate-to-severe rheumatoid arthritis who have had an insufficient response to conventional therapy with biologics or DMARDs. The analysis found that peresolimab was more efficacious than placebo at reducing the Disease Activity Score and inflammatory markers often elevated in rheumatoid arthritis. It also had a comparable safety profile to the placebo. However, the long-term safety profile of peresolimab use beyond 12 weeks remains unclear. The precise efficacy and safety profile of peresolimab is further limited by the study’s small sample size. Overall, this positive trial demonstrated the potential utility of peresolimab in treating rheumatoid arthritis.
Click here to read the study in NEJM
In-Depth [randomized controlled trial]: This was a phase double-blinded, randomized, placebo-controlled trial evaluating the safety and efficacy of peresolimab amongst adults with moderate-to-severe active rheumatoid arthritis. The primary outcome of interest was change in Disease Activity Score for 28 joints based on the C-reactive protein level (DAS28-CRP) between baseline and week 12. Additional outcomes of interest included percentage of patients with improvements from their baseline as calculated by the American College of Rheumatology (ACR) 20, 50, and 70 responses by the 12th week. Adult participants were included if they were diagnosed with moderate-to-severe active rheumatoid arthritis a minimum of three-months before screening, and had active synovitis with inadequate response to at least one conventional DMARD. Participants were excluded if they did not demonstrate any response to more than two biologics or targeted DMARDs. After applying inclusion and exclusion criteria, 98 patients were randomly assigned in a 2:1:1 ratio to receive 700mg (n=49) or 300mg (n=25) of peresolimab, or a placebo (n=24). Primary results of the analysis found that the 700mg peresolimab group demonstrated a significantly different DAS28-CRP score compared to placebo (difference in change, -1.09; 95% confidence interval, -1.73-0.46; p<0.001). Results of secondary analysis found that with respect to the ACR responses, the 700mg dose was superior to the placebo in improving 20% from baseline, but had no difference in improvements of 50% or 70% from baseline. There were no significant differences in incidence of adverse events between the groups. In summary, this study found that stimulation of the PD-1 inhibitory pathway through peresolimab may reduce disease severity and markers of inflammation amongst those with moderate-to-severe rheumatoid arthritis.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.