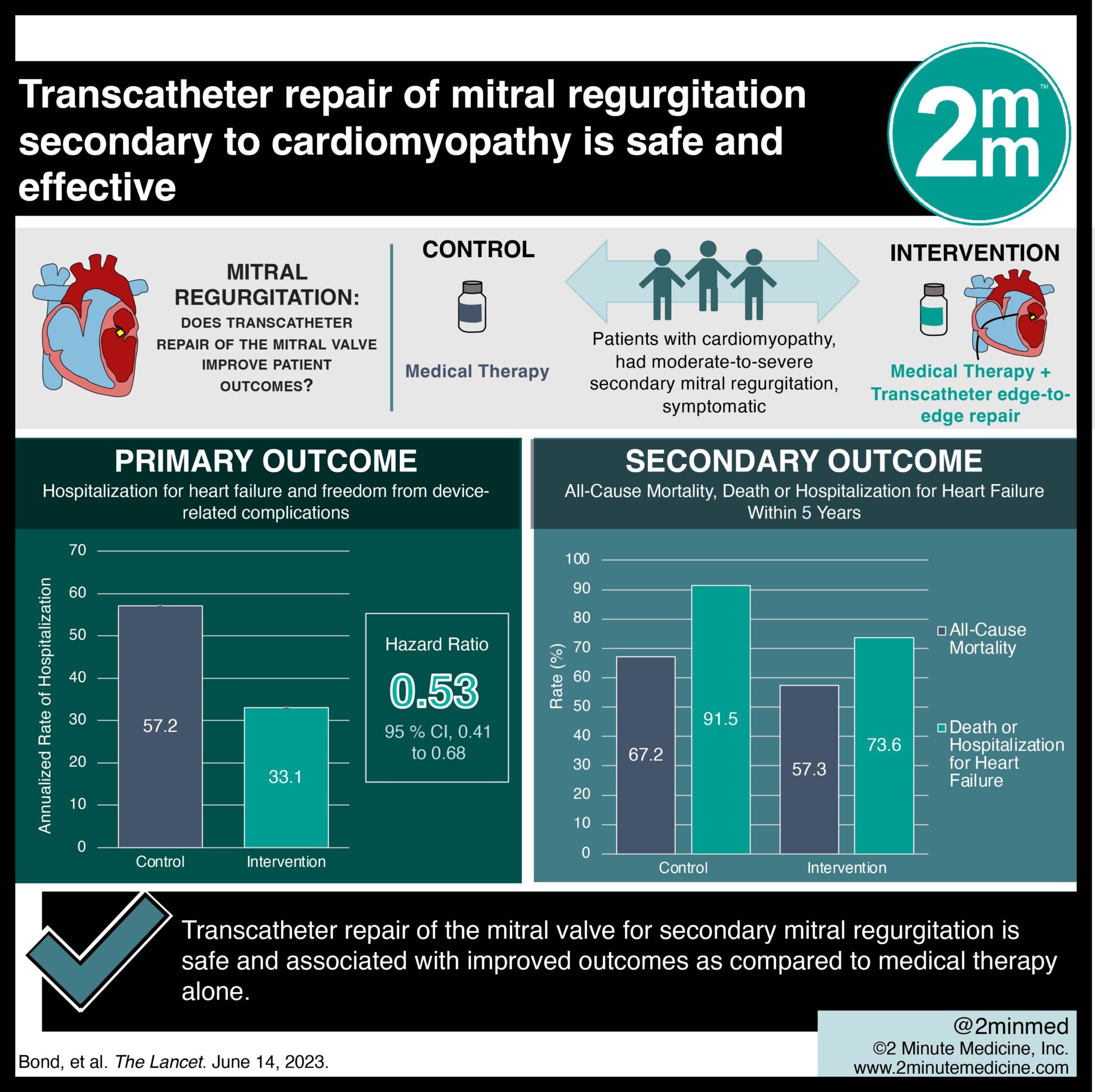
1. In this randomized controlled trial, patients who received transcatheter repair of mitral regurgitation secondary to cardiomyopathy had good safety outcomes as compared to medical therapy alone.
2. Patients who received transcatheter repair of secondary mitral regurgitation had a lower hospitalization rate than those who received medical therapy alone.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Mitral regurgitation is a common consequence of cardiomyopathy and can be associated with a poor prognosis, an increased rate of hospitalization for heart failure, and reduced survival. Transcatheter edge-to-edge repair of the mitral valve has been shown to reduce mitral regurgitation. However, there is a gap in knowledge as to whether this repair technique would be safe, effective, and sustained over a long-term follow-up period as compared to medical therapy alone in patients with heart failure and secondary mitral regurgitation. Overall, this study found that patients with heart failure and moderate-to-severe secondary mitral regurgitation who remained symptomatic despite maximal medical therapy did benefit from transcatheter repair of the mitral valve, which was safe and effective and led to prolonged survival during five years of follow-up. This study was limited by the fact that the device treatment was unblinded, and withdrawal from the trial by more patients in the control group than in the device group. Nevertheless, these study’s findings are significant, as they demonstrate that transcatheter repair of secondary mitral regurgitation is safe and sustainable, leading to improved survival over five years as compared to medical therapy alone.
Click to read the study in NEJM
Relevant Reading: Transcatheter Repair for Patients with Tricuspid Regurgitation
In-Depth [randomized controlled trial]: This randomized, parallel-controlled, open-label trial was conducted with the MitraClip (Abbott) to evaluate transcatheter edge-to-edge repair in patients with severe mitral regurgitation and heart failure. Patients who had ischemic or nonischemic cardiomyopathy with a left ventricular ejection fraction of 20 to 50%, had moderate-to-severe (3+) or severe (4+) secondary mitral regurgitation that was confirmed at an echocardiographic core laboratory before enrollment, and remained symptomatic despite the use of stable maximal doses of guideline-directed medical therapy were eligible for the study. Patients who had a left ventricular end-systolic dimension of more than 7cm, severe pulmonary hypertension, and moderate or severe symptomatic right ventricular failure were excluded from the study. The primary outcome measured was hospitalization for heart failure and freedom from device-related complications. Outcomes in the primary analysis were assessed via the intention-to-treat protocol with Kaplan-Meier analysis and Cox regression. Based on the primary analysis, the annualized rate of hospitalization for heart failure through three years was 33.1% per year in the device group and 57.2% per year in the control group (Hazard Ratio [HR], 0.53; 95% confidence interval [CI], 0.41 to 0.68). All-cause mortality through five years was 57.3% in the device group and 67.2% in the control group (HR, 0.72; 95% CI, 0.58 to 0.89). Death or hospitalization for heart failure within five years occurred in 73.6% of the patients in the device group and in 91.5% of those in the control group (HR, 0.53; 95% CI, 0.44 to 0.64). This study demonstrates that transcatheter repair of the mitral valve for secondary mitral regurgitation is safe and associated with improved outcomes as compared to medical therapy alone.
Click here to read this study in NEJM.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.