2. There were no significant differences between groups for overall survival.
Evidence Rating Level: 1 (Excellent)
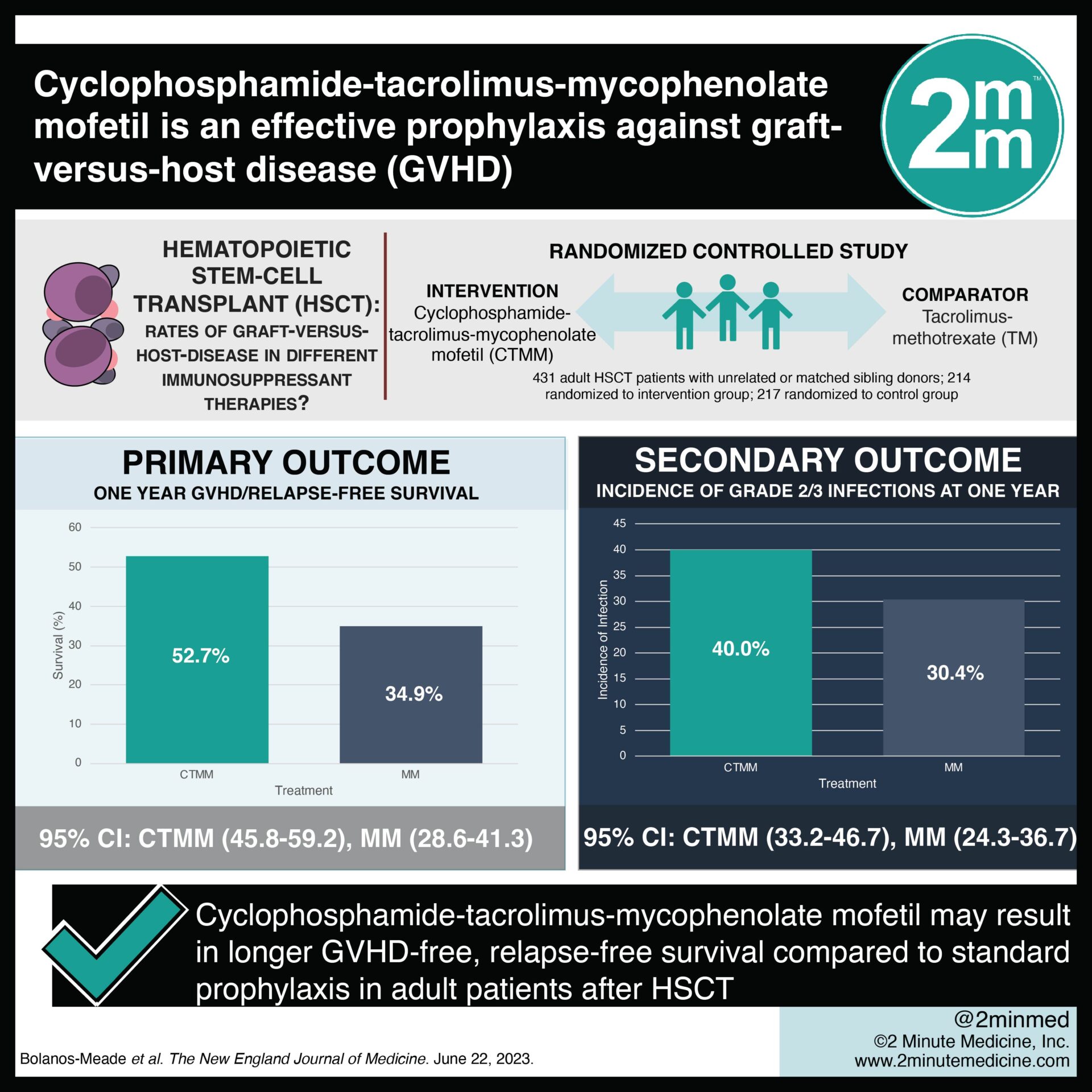
Study Rundown: GVHD is a major complication of HSCT. The combination of immunosuppressants methotrexate and a calcineurin inhibitor form the standard prophylactic treatment for GVHD. Recent trials have identified cyclophosphamide-tacrolimus-mycophenolate mofetil as a promising alternative. In this phase three randomized clinical trial, adult patients undergoing their first HSCT with unrelated or matched sibling donors received either cyclophosphamide-tacrolimus-mycophenolate mofetil (experimental prophylaxis) or tacrolimus-methotrexate (standard prophylaxis). Patients were followed for 12 months. The primary outcome was GVHD-free, relapse-free survival at one year. The primary outcome occurred in a significantly higher proportion of patients in the experimental prophylaxis group compared to the standard prophylaxis group. For secondary outcomes, grade II to IV acute GVHD at day 100, one-year overall survival, disease-free survival, and incidence of transplantation-related death were not significantly different between groups. The incidence of chronic GVHD was significantly lower in the experimental prophylaxis group. As limitations, subgroup outcome analyses for related, unrelated, and HLA-mismatched could not be completed due to sample size constraints. Study results may not be generalizable to all ethnicities, as the sample size for non-White patients was low. Overall, cyclophosphamide-tacrolimus-mycophenolate mofetil was associated with lower rates of GVHD in HSCT patients in this study.
In-Depth [randomized controlled trial]: In this randomized clinical trial, adult patients undergoing HSCT with unrelated or matched sibling donors (n=431) were randomized to receive either cyclophosphamide-tacrolimus-mycophenolate mofetil (n=214) or tacrolimus methotrexate (n=217). The primary outcome was GVHD-free, relapse-free survival at one year. A total of 98 and 135 primary outcome events occurred in the experimental prophylaxis and standard prophylaxis groups, respectively. The experimental prophylaxis group had a significantly lower risk of grade III or IV acute GVHD, chronic GVHD, disease relapse or progression, or death as estimated by a multivariate Cox regression model (estimated hazard ratio, 0.64; 95% Confidence Interval [CI], 0.49 to 0.83; p=0.001). The one-year adjusted GVHD-free, relapse-free survival was 52.7% (95% CI, 45.8 to 59.2) for the experimental prophylaxis group and 34.9% (95% CI, 28.6 to 41.3) for the standard prophylaxis group. For secondary outcomes, estimated one-year overall survival, disease-free survival, and incidence of transplantation-related death were not significantly different between groups. Immunosuppression-free survival at one year was higher in the experimental prophylaxis group (50%; 95% CI, 42.8 to 57.2) compared to the standard prophylaxis group (39.7%; 95% CI, 32.9 to 46.8). Infections occurred in 52.4% of the experimental prophylaxis group compared to 47.2% of the standard prophylaxis group. There were between-group differences in the incidence of grade two or three infections at one year (experimental, 40.0%; 95% CI, 33.2 to 46.7) and standard, 30.4% (95% CI, 24.3 to 36.7). This study provided evidence that cyclophosphamide-tacrolimus-mycophenolate mofetil prophylaxis may result in longer GVHD-free, relapse-free survival compared to standard prophylaxis for adult patients after HSCT.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.








