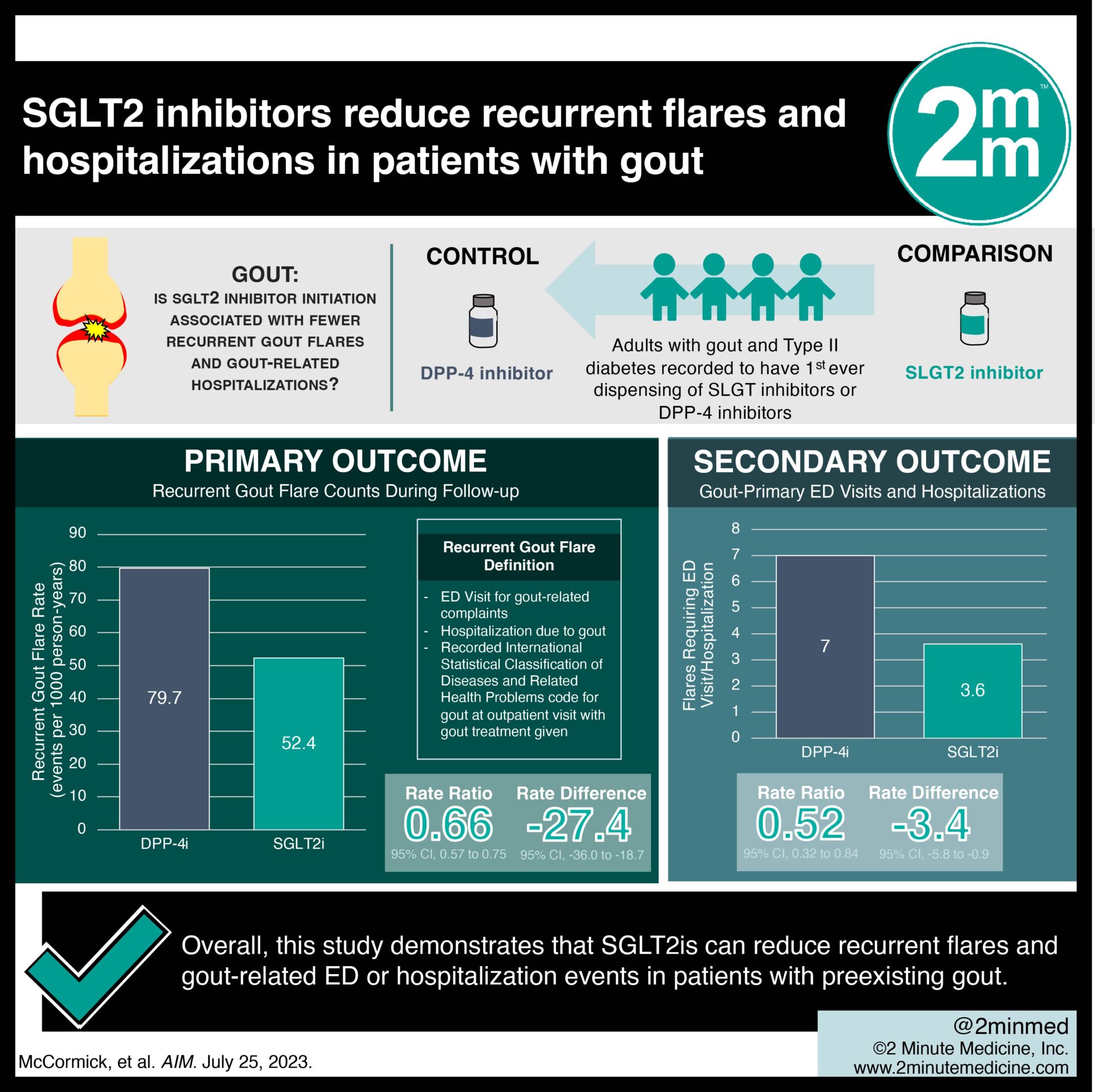
- In this population cohort study, patients with gout who used sodium-glucose cotransporter-2 inhibitors (SGLT2is) experienced fewer recurrent flares than those using dipeptidyl peptidase 4 inhibitors (DPP-4is).
- Those who used SGLT2 inhibitors also presented to the emergency department (ED) less frequently for gout-related visits than those using DPP-4is.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Gout is a common inflammatory arthritis that causes recurrent flares significantly impacting patient quality of life. These flares have also been associated with increased rates of opioid use and ED visits in patients with gout. SGLT2is have been shown to reduce serum urate levels, and their use has been previously associated with a decreased risk of developing gout. However, there is a knowledge gap in understanding the effect of SGLT2is on patients already with gout as well as whether using SGLT2is helps prevent recurrent flares. The present population cohort study assessed gout flares and hospitalizations in patients using SGLT2is and DPP-4is. Overall, this study found that using SGLT2is was associated with a lower risk of recurrent gout flares and gout-primary ED visits and hospitalizations in patients with gout. This study was limited by not capturing flares that did not require medical attention. There was also a potential risk identified for residual unmeasured confounding. Nevertheless, these study’s findings are significant, as they demonstrate that SGLT2i initiation is associated with a lower risk of developing recurrent gout flares and gout-related hospitalization among patients with pre-existing gout.
Click to read the study in AIM
Relevant Reading: Assessing the Risk for Gout With Sodium–Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes
In-Depth [retrospective cohort study]: This retrospective study sourced the data from the population of British Columbia in Canada for a propensity score-matched, new user cohort study. Patients aged 18 years or older with gout and type two diabetes that were recorded to have their first-ever dispensing of SGLT2is or DPP-4is between January 1st, 2014 and June 30th, 2022 were eligible for the study if they had at least one year of continuous enrollment. Patients who did not fit these inclusion criteria were excluded from the study. The primary outcome measured was recurrent gout flare counts during follow-up. This was defined as either an ED visit for gout-related complaints, hospitalization due to gout, or a recorded International Statistical Classification of Diseases and Related Health Problems (ICD) code for gout at an outpatient visit with gout treatment given. Outcomes in the primary analysis were assessed via a sequential time-stratified propensity score-matched analysis. Based on the primary analysis, the flare rate was lower among SGLT2i initiators than DPP-4i initiators (52.4 and 79.7 events per 1000 person-years, respectively), with a rate ratio (RR) of 0.66 (95% Confidence Interval [CI], 0.57 to 0.75) and a rate difference (RD) of -27.4 (95% CI, -36.0 to -18.7) per 1000 person-years. The corresponding RR and RD for gout-primary ED visits and hospitalizations were 0.52 (95% CI, 0.32 to 0.84) and -3.4 (95% CI, -5.8 to -0.9) per 1000 person-years, respectively. In summary, this study demonstrates that SGLT2is can reduce recurrent flares and gout-related ED or hospitalization events in patients with preexisting gout.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.