2. Patients assigned to atezolizumab had fewer treatment-related adverse events and treatment-related deaths.
Evidence Rating Level: 1 (Excellent)
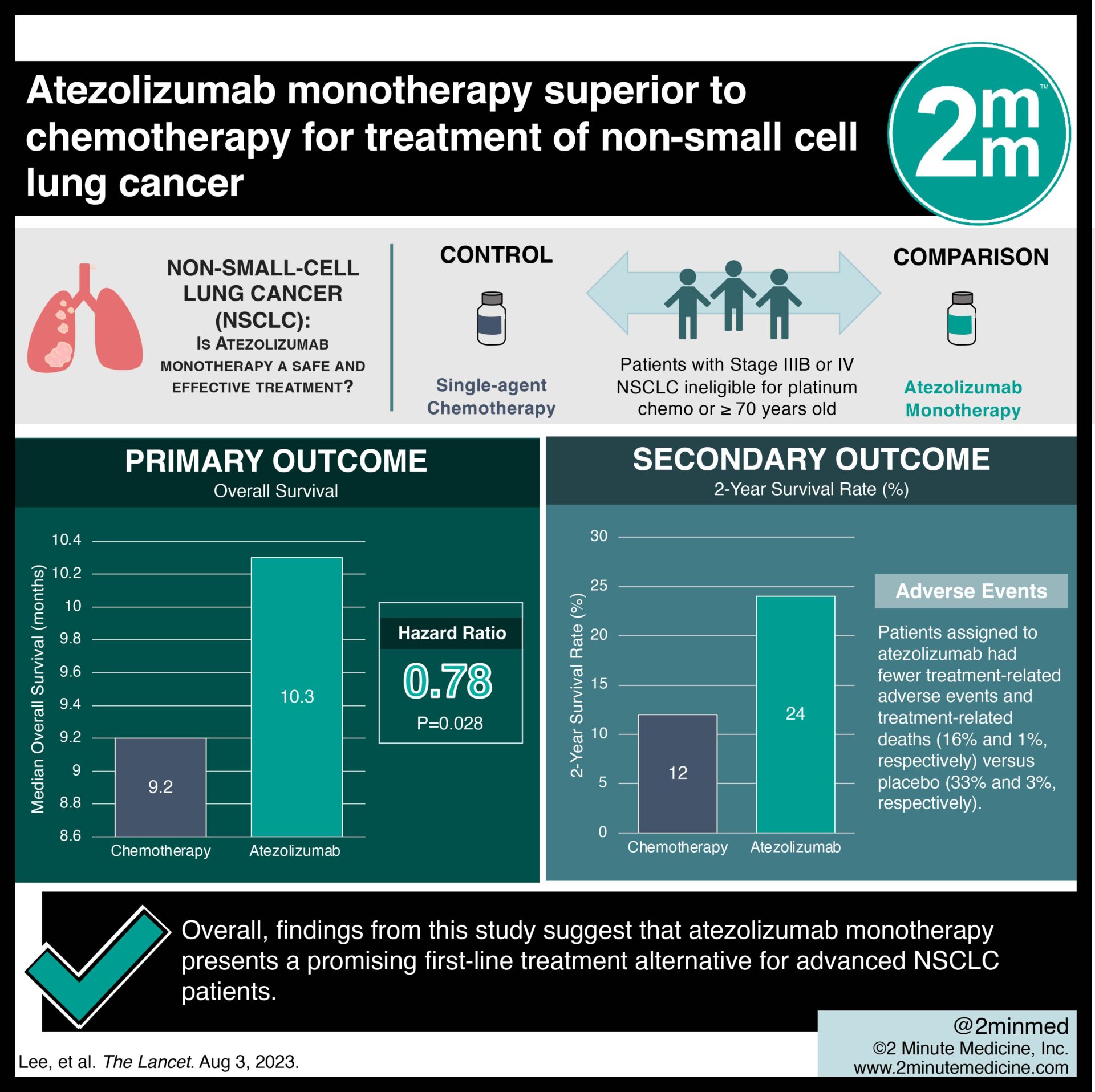
Study Rundown: Prior first-line trials in advanced non-small-cell lung cancer (NSCLC) lacked representation for older or patients with reduced fitness. Data surrounding treatment safety and efficacy for advanced NSCLC remains scarce. This randomized controlled trial aimed to compare the safety and efficacy of atezolizumab monotherapy with single-agent chemotherapy for patients with NSCLC who were ineligible for platinum-based chemotherapy. The primary outcome of this study was improved overall survival with atezolizumab compared to chemotherapy, while key secondary outcomes included enhanced quality of life and fewer adverse events with atezolizumab. According to study results, atezolizumab was associated with a significant mortality benefit along with quality-of-life preservation and a favorable safety profile. Although this study was well done, it was limited by selection bias, thus skewing the validity of the results.
Click to read the study in The Lancet
Relevant Reading: First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer
In-depth [randomized-controlled trial]: Between Sept 11, 2017, and Sept 23, 2019, 843 patients were screened for eligibility across 91 sites in 23 countries. Included were patients with stage IIIB or IV NSCLC ineligible for platinum-based chemotherapy or those ≥ 70 years old with ECOG 0-1 with substantial comorbidities. Altogether, 453 patients (302 to atezolizumab and 151 to chemotherapy) were included in the final analysis. The primary outcome of overall survival was greater in atezolizumab (median overall survival 10.3 months, 95% confidence interval [CI] 9.4-11.9) compared to chemotherapy (median overall survival 9.2 months, 95% CI 5.9-11.2, stratified hazard ratio [HR] 0.78, p=0.028). The 2-year survival rate with atezolizumab was 24% (95% CI 19.3-29.4) versus 12% (95% CI 6.7-18.0) with chemotherapy. Patients assigned to atezolizumab had fewer treatment-related adverse events and treatment-related deaths (16% and 1%, respectively) versus placebo (33% and 3%, respectively). Overall, findings from this study suggest that atezolizumab monotherapy presents a promising first-line treatment alternative for advanced NSCLC patients.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.








