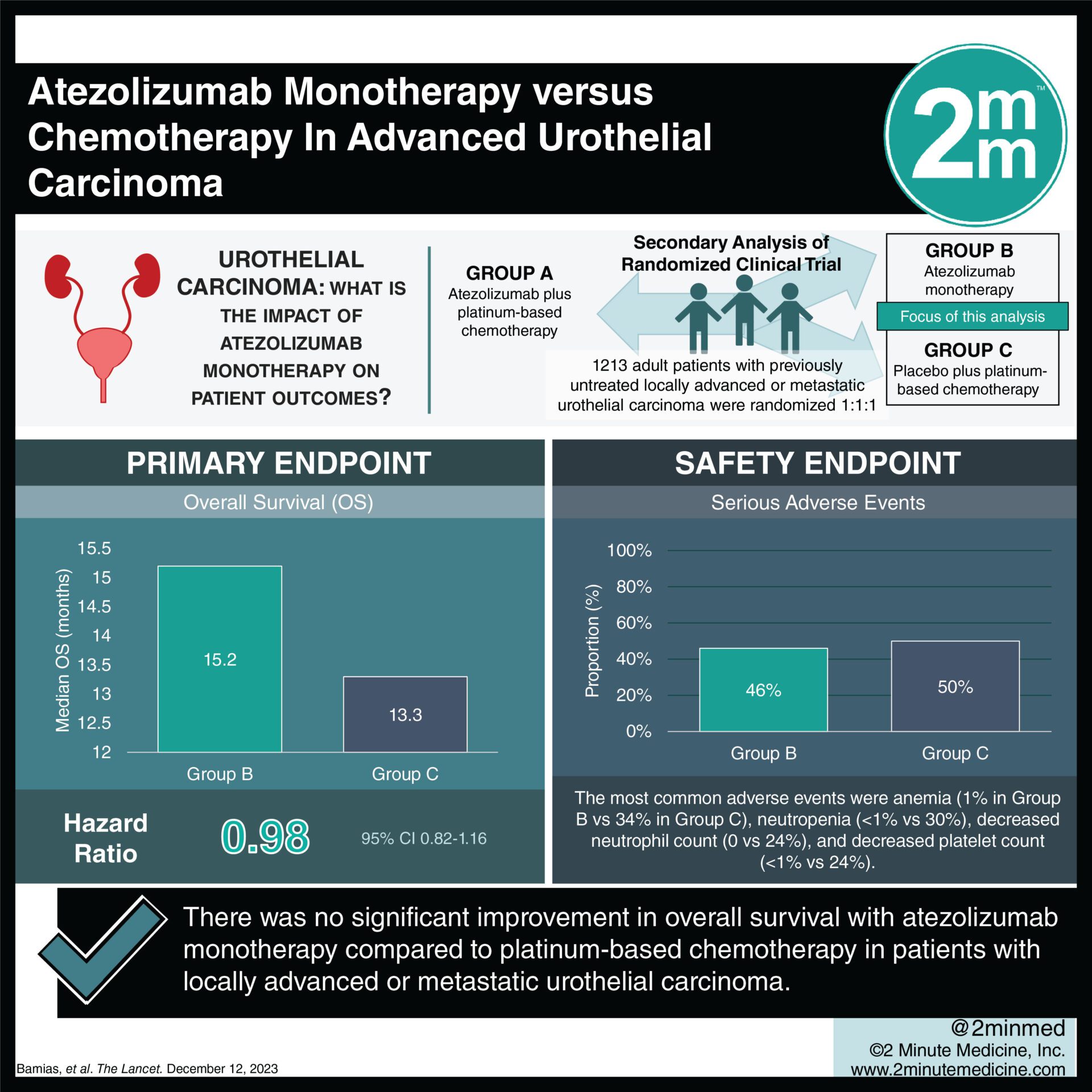
In a study comparing atezolizumab monotherapy with platinum-based chemotherapy in advanced urothelial carcinoma, overall survival did not significantly differ in the general population, but improved outcomes were observed in patients with high PD-L1 expression ineligible for cisplatin.
1. The median overall survival was similar between both groups, 15.2 months in atezolizumab monotherapy vs 13.3 months in chemotherapy, with HR 0.98.
2. Atezolizumab monotherapy was better tolerated, with 16% experiencing grade 3–4 treatment-related adverse events compared to 80% in chemotherapy.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Cisplatin-based chemotherapy is the standard of care for advanced urothelial carcinoma, but due to ineligibility in some patients, alternative approaches were studied. A previous study investigated the addition of immunotherapy to chemotherapy, but the results did not improve overall survival, however, comparison in some subgroups found significant improvement. This study reports the final analysis of atezolizumab monotherapy vs chemotherapy in advanced urothelial carcinoma. The primary endpoint was overall survival (OS), and secondary endpoints included objective response rate (ORR), duration of response (DoR), quality of life (QoL), and safety. Median OS was 15.2 months in group B and 13.3 months in group C, with HR 0.98. In patients who were ineligible for cisplatin and had high PD-L1 expression, the median OS was 18.6 months in group B vs 10.0 months in group C, with HR 0.56. ORR was 24.2% in group B and 44.4% in group C for the overall population and was 40.0% in group B and 32.6% in group C in patients ineligible for cisplatin with high PD-L1 expression. In those with a confirmed response, the median DoR was 29.6 months in group B versus 8.1 months in group C. QoL from surveys were similar between both groups. With regards to safety, grade 3–4 treatment-related adverse events occurred in 16% of group B and 80% of group C, with the most common events being anemia (1% vs 34%), neutropenia (<1% vs 30%), decreased neutrophil count (0 vs 24%), and decreased platelet count (<1% vs 24%). The strengths of this study included its sample size and follow-up period, and the limitations included multiple protocol amendments. Overall, there was no improvement found in the general population when comparing atezolizumab monotherapy versus platinum-based chemotherapy in advanced urothelial cancer but there were some improved outcomes in patients with high PD-L1 expression who were also ineligible for cisplatin.
Click to read the study in Lancet
Relevant Reading: Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial
In-Depth [randomized controlled trial]: This international, partially blinded phase 3 trial randomized adults with previously untreated locally advanced or metastatic urothelial carcinoma into 3 groups (1:1:1), atezolizumab plus platinum-based chemotherapy (group A), atezolizumab monotherapy (group B, 362 patients), or placebo plus platinum-based chemotherapy (group C, gemcitabine + cisplatin/carboplatin, 400 patients). This study focused on comparing groups B and C. Median follow-up was 13.4 months. Median OS was 15.2 months (95%CI, 13.1-17.7) in group B and 13.3 months (95%CI, 11.9-15.6) in group C, with HR 0.98 (95%CI, 0.82-1.16). In patients who were ineligible for cisplatin and had high PD-L1 expression, the median OS was 18.6 months (95%CI, 14.0-49.4) in group B vs 10.0 months (95%CI, 7.4-18.1) in group C, with HR 0.56 (95%CI, 0.34-0.91). ORR was 24.2% (95%CI, 19.9-29.0) in group B and 44.4% (95%CI, 39.2-49.7) in group C for the overall population, and was 40.0% in group B and 32.6% in group C in patients ineligible for cisplatin with high PD-L1 expression. In those with a confirmed response, the median DoR was 29.6 months (95%CI, 15.9-NA) in group B versus 8.1 months (6.3-8.5) in group C. QoL from surveys were similar between both groups. With regards to safety, grade 3–4 treatment-related adverse events occurred in 16% of group B and 80% in group C, with the most common events being anemia (1% vs 34%), neutropenia (<1% vs 30%), decreased neutrophil count (0 vs 24%), and decreased platelet count (<1% vs 24%). Overall, there was no improvement found in the general population when comparing atezolizumab monotherapy versus platinum-based chemotherapy in advanced urothelial cancer but there were some improved outcomes in patients with high PD-L1 expression who were also ineligible for cisplatin.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.