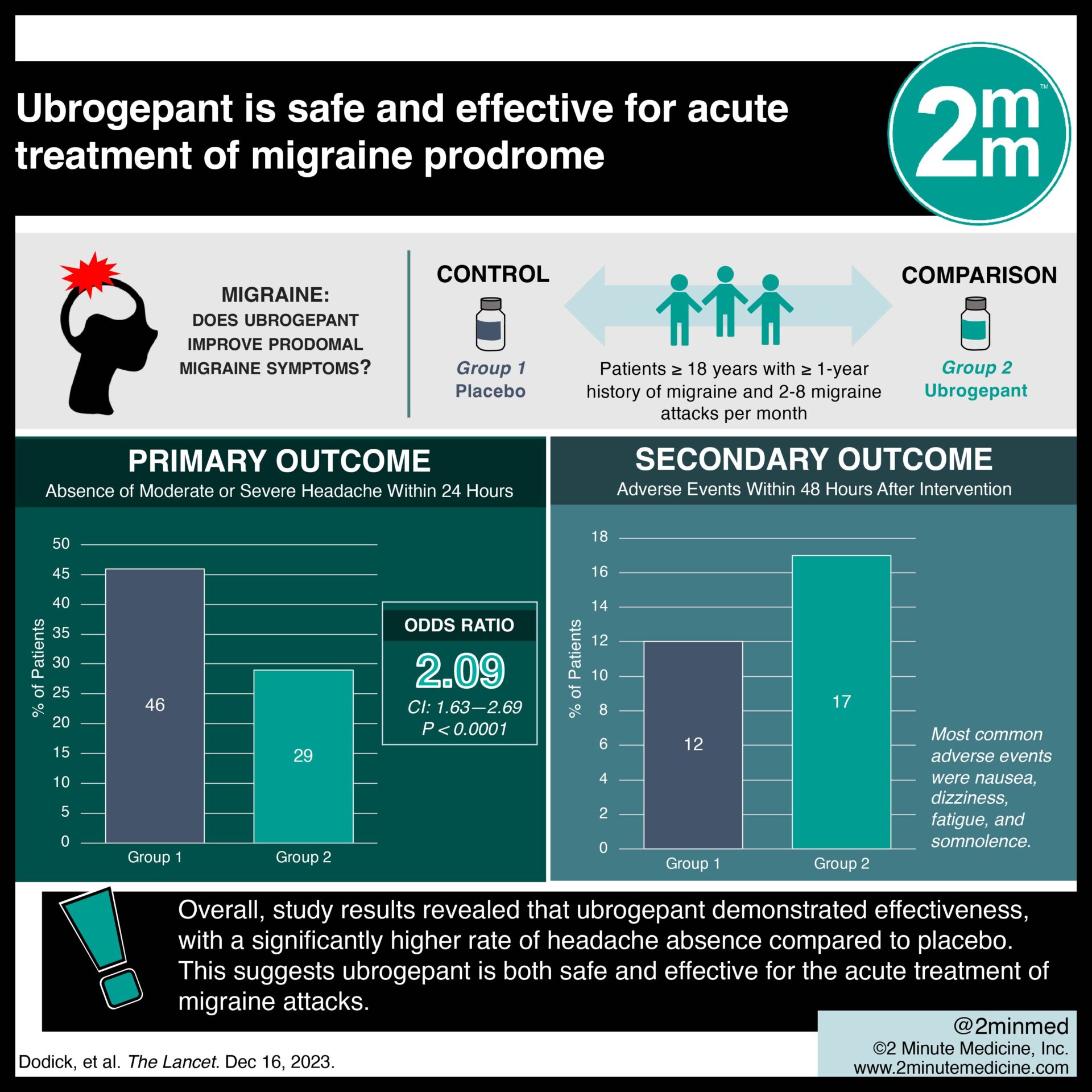
1. Absence of moderate or severe headache 24 hours following administration of treatment drug was significantly greater among patients with migraine attacks that were treated with ubrogepant during the prodrome phase.
2. Adverse events were slightly more frequent in the ubrogepant group versus placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Ubrogepant, a calcitonin gene-related peptide (cGRP) receptor antagonist, is approved for acute migraine treatment. However, its use during the prodromal phase to reduce the frequency of subsequent migraine headaches is not well understood. This phase 3 randomized controlled trial aimed to evaluate the safety, efficacy, and tolerability of ubrogepant when administered during the prodrome phase of a migraine attack. The primary outcome was the absence of moderate or severe headache within 24 hours after drug use, while the key secondary outcome was the absence of any headache. According to study results, ubrogepant demonstrated effectiveness, with a significantly higher rate of headache absence compared to placebo. This study was strengthened by individuals of all age groups with a chronic history of migraines, thus adding to the validity of the findings.
Click to read the study in The Lancet
Relevant Reading: Ubrogepant for the Treatment of Migraine
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.