Enzalutamide combined with androgen deprivation therapy (ADT) seemed to be a superior treatment regimen in Black men with metastatic hormone-sensitive prostate cancer (mHSPC) versus ADT plus standard treatment, researchers reported.
In a multicenter phase II trial, 94% of the patients (n=30 of 32, 95% CI 80% to 98%) taking enzalutamide achieved the 7-month prostate-specific antigen (PSA) response rate (SMPR) versus 65% (17 of 26, 95% CI 46% to 81%) taking bicalutamide (P=0.008), for a 29% difference (95% CI 5% to 50%), according to Ulka N. Vaishampayan, MD, of the University of Michigan in Ann Arbor, and co-authors.
Additionally, Black patients had an increased likelihood of benefit with enzalutamide compared with bicalutamide with SMPRs of 93% (13 of 14, 95% CI 69% to 99%) versus 42% (five of 12, 95% CI 19% to 68%, P=0.009), respectively, they wrote in JAMA Network Open.
“In this study, Black men with mHSPC had a decreased response rate with ADT and bicalutamide,” they stated. “The incorporation of enzalutamide in mHSPC therapy overcame the disparity in biochemical response rate and OS [overall survival] outcomes between Black and non-Black patients.”
However, Vaishampayan’s group explained that the study was closed administratively slightly short of the target sample size after results from the LATITUDE trial were released. The latter reported that abiraterone and prednisone improved OS in mHSPC, so Vaishampayan and co-authors said they could no longer ethically randomize patients to receive bicalutamide at that point.
They also noted that other studies — STAMPEDE, TITAN, ENAZMET, and ARCHES — offered “Multiple ways of enhancing and optimizing therapy in hormone-sensitive disease,” but “the populations enrolled were predominantly White,” so the findings may not apply to Black patients with advanced prostate cancer who “have specific nuances of presentation, prognosis, and therapy outcomes.”
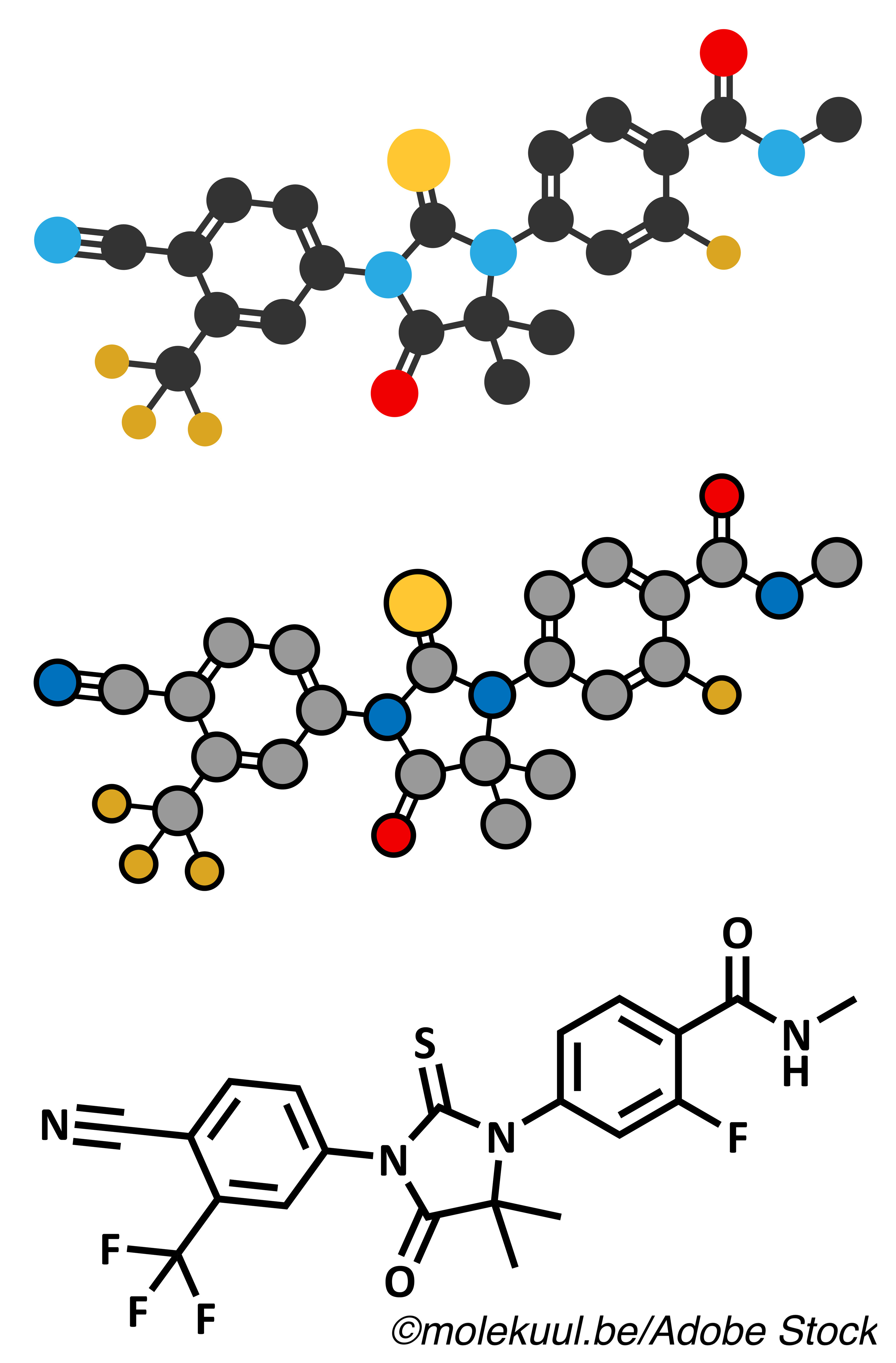
The trial compared outcomes of ADT plus bicalutamide versus ADT plus enzalutamide among White and Black men. Enzalutamide is a second-generation androgen receptor inhibitor that blocks nuclear translocation and DNA binding in prostate cancer cells, the authors explained. The agent was FDA-approved in 2019 for patients with metastatic castration-sensitive prostate cancer (mCSPC), based on result of ARCHES, and a year earlier for patients with castration-resistant prostate cancer.
In an invited commentary, Neeraj Agarwal, MD, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City, and co-authors noted that “What stands out and makes this report so remarkable is that it is a prospective randomized clinical trial of men with mHSPC with a novel hormonal therapy where subset analysis of outcomes in Black men was prespecified.”
“Even though the trial was not powered to compare outcomes within race, it is interesting that treatment outcomes within Black patients compared with non-Black patients were significantly different,” they added.
Nonetheless, Agarwal and colleagues pointed out that unanswered questions remain in terms of why Black men with prostate cancer have higher mortality rates versus non-Black men, but similar outcomes with systemic therapy for metastatic disease.
“Ultimately, racial disparities in prostate cancer will be better understood with greater emphasis on enrollment of Black men in clinical trials across disease stages,” they stated.
The current study was conducted at several national treatment centers and had a primary endpoint of 7-month PSA response rate (SMPR) in each group, which was used as a surrogate for OS. Secondary endpoints included comparing the primary endpoint by race (Black vs non-Black), response rates, adverse events (AEs) in each group, and time to event endpoints — time to PSA progression (TTPP) and OS — by treatment group and by race.
Eligible patients (n=71; median age 65) had histologically confirmed prostate adenocarcinoma, radiological evidence of metastases, a minimum PSA level of 4 ng/mL, no history of seizures, and adequate marrow, renal, and liver function. They were stratified by race (Black or other) and bone pain (present or absent), and all had metastatic biopsy collection performed.
Black men made up 41% of the study population while 58% were White and 1% were Asian. Over one-third (37%) expressed bone pain, and more than half (52%) presented with extensive disease. The median baseline PSA level was 56.3 ng/mL in the enzalutamide group and 60 ng/mL in the bicalutamide group. Participants were randomized 1:1 to receive oral enzalutamide (160 mg daily) or bicalutamide (50 mg daily) in addition to ADT.
Vaishampayan’s group reported that non-Black patients had comparable the SMPR rates of 94% (95% CI 74% to 99%) with enzalutamide and 86% (95% CI 60% to 96%) with bicalutamide. The 12-month PSA response rates were 84% and 34%, respectively.
No grade 4 treatment-related AEs were observed in either group, and the TTPP and OS data are immature, so follow-up is ongoing, according to the authors.
In the meantime, “ADT plus bicalutamide is inadequate therapy in all cases, but especially for Black patients,” they stated. “Enzalutamide demonstrated a greater magnitude of improvement over bicalutamide, in the rate and duration of PSA response in Black patients.”
-
Enzalutamide paired with androgen deprivation therapy (ADT) was associated with improved outcomes versus bicalutamide plus ADT in Black men with metastatic hormone-sensitve prostate cancer (mHSPC).
-
The 7-month prostate-specific antigen (PSA) response rate was significantly improved with enzalutamide versus bicalutamide plus ADT among Black patients.
Shalmali Pal, Contributing Writer, BreakingMED™
The study was funded by Astellas Pharma Global Development, which provided the enzalutamide, and the NIH.
Vaishampayan reported support from, and/or relationships with, Astellas, Bristol Meyers Squibb (BMS), AAA, Alkermes, Eisai, Exelixis, Sanofi, Bayer, Merck, and Pfizer. Co-authors reported support from, and/or relationships with, Sanofi, Dendreon, Bayer, Janssen, BMS, Genentech, EMD Serono, Merck, Seattle Genetics/Astellas, AstraZeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Physicians Education Resource, Onclive, Research to Practice, Medscape, UptoDate, Bavarian Nordic, Seattle Genetics, QED (uncompensated), and Debiopharm.
Agarwal reported support from, and/or relationships with, Astellas, AstraZeneca, Bayer, BMS, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Janssen, Merck, Nektar, Novartis, Pfizer, Pharmacyclics, Seattle Genetics, Bavarian Nordic, Calithera, Celldex, GlaxoSmithKline, Immunomedics, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Takeda, and Tracon. A co-authors reported support from, and/or relationships with, Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, Sanofi, Xencor, Genentech/Roche, Seattle Genetics, Incyte, Tricon Pharmaceuticals, Peleton Therapeutics, and Pfizer.
Cat ID: 25
Topic ID: 78,25,730,25,192,73,925



Create Post
Twitter/X Preview
Logout