2. Grade 3-4 adverse events were more common in the nivolumab group, however, there were no reported deaths.
Evidence Rating Level: 1 (Excellent)
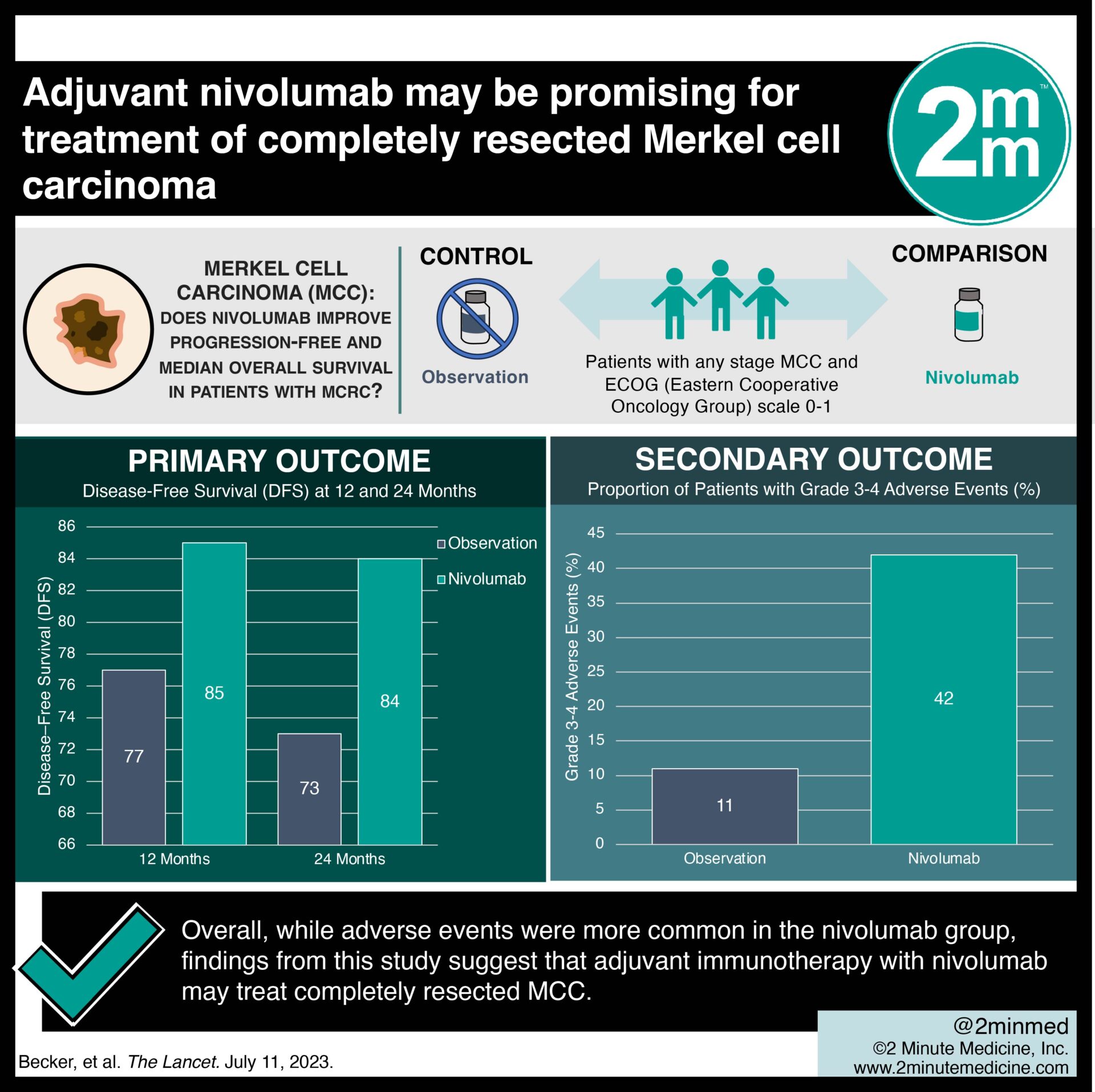
Study Rundown: Merkel cell carcinoma (MCC), an aggressive skin cancer, poses a clinical challenge even after complete resection and radiation therapy. Immune checkpoint inhibitors targeting PD-1 and PD-L1 have shown promise in advanced MCC. This randomized controlled trial aimed to assess the safety and effectiveness of adjuvant immune checkpoint inhibition using nivolumab in completely resected MCC. The primary outcome was landmark disease-free survival (DFS) at 12 and 24 months, while key secondary outcome was safety and grade 3-4 adverse events. According to study results, median disease-free survival was greater in the nivolumab group compared to observation and median-free survival was not met in either group. Although this study was well done, it was limited by the relatively short follow-up period for overall survival outcomes.
Click to read the study in The Lancet
Relevant Reading: Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer
In-depth [randomized-controlled trial]: Between Oct 1, 2014, and Aug 31, 2020, 219 patients were screened for eligibility across 20 academic medical centers in Germany and the Netherlands. Included were patients with any stage MCC and an Eastern Cooperative Oncology Group (ECOG) scale 0-1. Altogether, 179 patients (118 in nivolumab and 61 in observation) were included in the final analysis. The primary outcome of DFS was greater in the nivolumab group at 12 and 24 months (85% and 84%, respectively) than in the observation group (77% and 73%, respectively). Median DFS was not reached in either group (between-groups hazard ratio 0.58, 95% confidence interval [CI] 0.30-1.12) at 24.3 months. Secondary outcomes revealed that grade 3–4 adverse events were higher in nivolumab (42%) than observation only (11%), but no treatment-related deaths occurred. Overall, findings from this study suggest that adjuvant immunotherapy with nivolumab may treat completely resected MCC.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.














