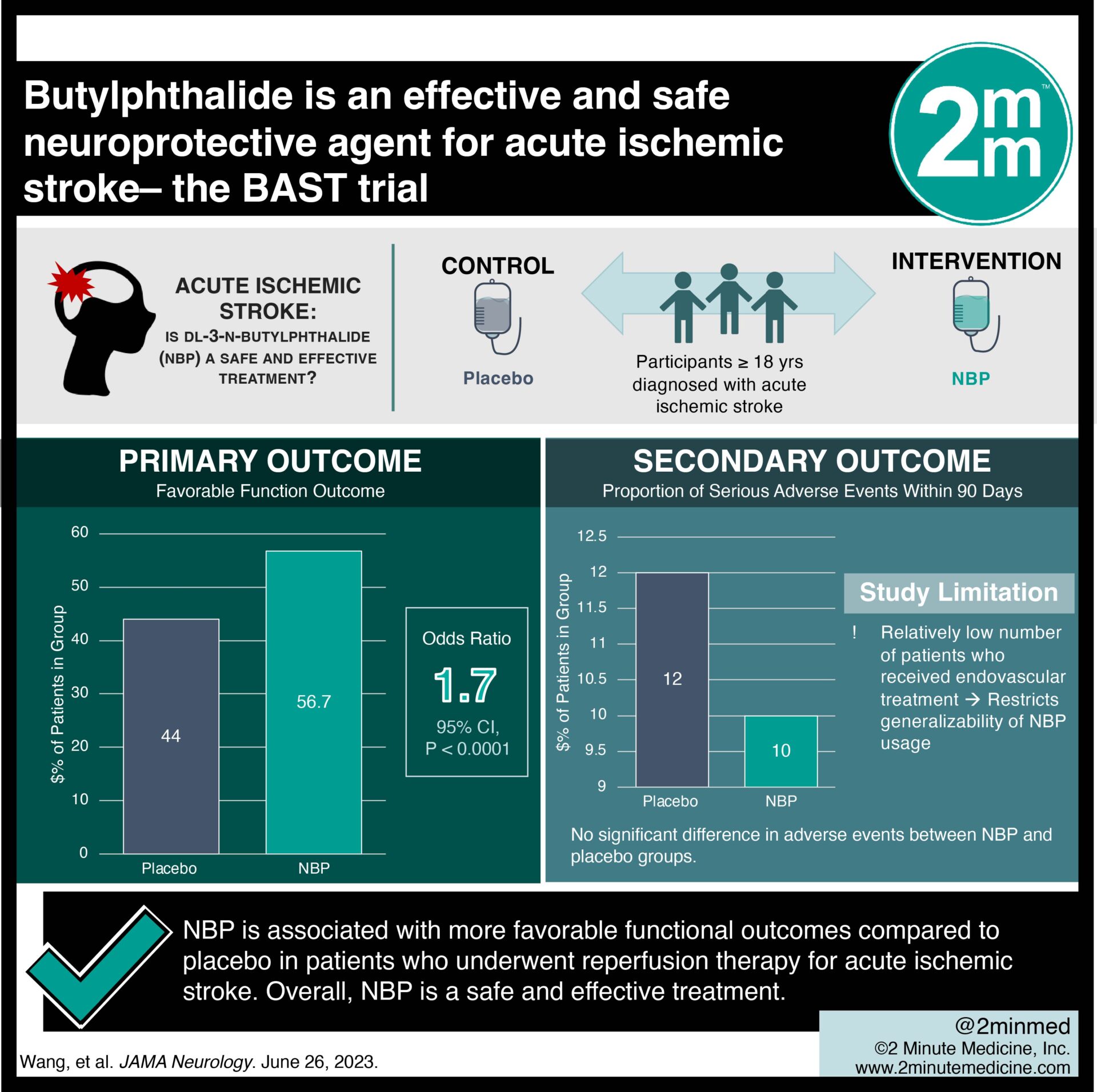
1. Butylphthalide is effective — the BAST trial showed that butylphthalide is associated with more favorable functional outcomes compared to placebo in patients who underwent reperfusion therapy for acute ischemic stroke.
2. Butylphthalide is safe – the treatment and placebo group had similar incidences of serious adverse outcomes (death, symptomatic intracranial hemorrhage and other side effects).
Evidence Rating Level: 1 (Excellent)
Study Rundown: Although reperfusion therapy is the primary approach for treating ischemic stroke, unfortunately, a substantial proportion of patients do not experience its benefits in a timely manner. Consequently, exploring alternative and adjunctive treatments, such as neuroprotective medications that can slow the progression of ischemia and aid in neuron recovery, has become crucial in stroke management. The BAST clinical trial (Butylphthalide for Acute Ischemic Stroke Patients Receiving Intravenous Thrombolysis or Endovascular Treatment) aimed to evaluate the effectiveness and safety of DL-3-n-butylphthalide (NBP), which is one such neuroprotective drug. Employing random effect logistical regression, the study discovered that the use of NBP was associated with a higher proportion of favorable functional outcomes after 90 days compared to placebo. Moreover, there was no significant difference in adverse events between NBP and placebo groups. One limitation of the study was the relatively low number of patients who received endovascular treatment, which restricts the generalizability of NBP usage in individuals undergoing this reperfusion method. Nevertheless, in contrast to previous unsuccessful attempts with neuroprotective trials involving substances like albumin, uric acid, magnesium sulfate, and natalizumab, the BAST trial demonstrates that combining NBP with reperfusion therapy may yield improved functional outcomes for stroke patients.
Click to read the study in JAMA Neurology
Relevant Reading: Stroke Treatment Academic Industry Roundtable X: Brain cytoprotection therapies in the reperfusion era
In-Depth [randomized clinical trial]: This double-blind, placebo-controlled, randomized clinical trial was conducted in China from July 2018 to May 2022. The study enrolled participants 18 years or older diagnosed with acute ischemic stroke. Eligible participants started the trial drug within six hours of experiencing stroke symptoms and also had received reperfusion therapy. Exclusion criteria included individuals with a history of intracranial hemorrhagic diseases, dysphagia, hematologic disorders, and liver or kidney dysfunction. Additionally, those with a lower likelihood of poor functional outcomes based on ASPECTS score and those who had previously taken NBP were also excluded. Among the 1216 patients included in the study, a favorable functional outcome was observed in 56.7% of the NBP group compared to 44.0% of the placebo group. In other words, the NBP group had 1.7 times greater odds of experiencing favorable functional outcomes than the placebo group (p<0.001). Further analysis of prespecified subgroups, including age, sex, NIHSS score, comorbidities, and reperfusion method, demonstrated that NBP was superior to placebo for all patient categories. Notably, there was no significant difference in the proportion of serious adverse events within 90 days between the NBP group (10%) and the placebo group (12%).
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.