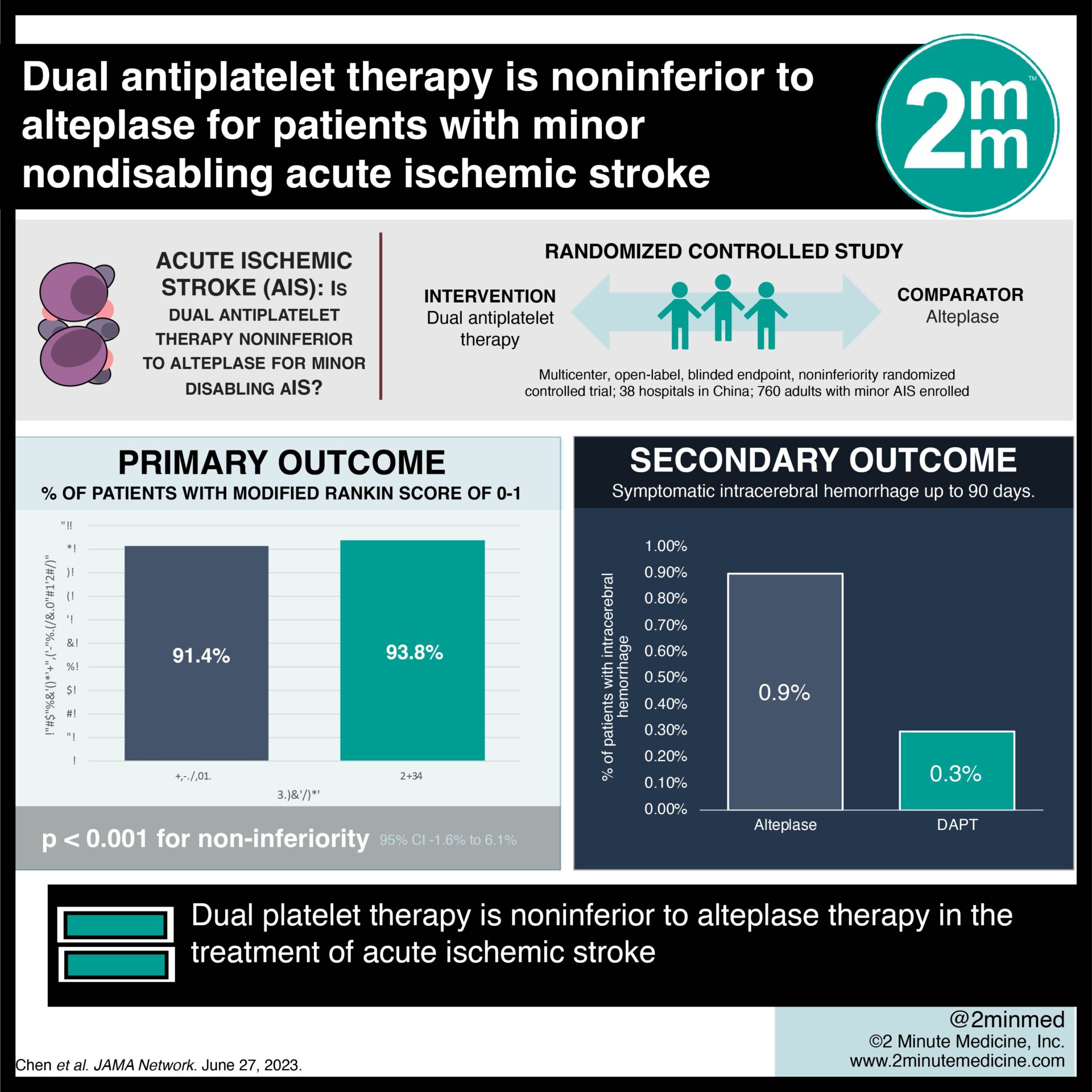
1. The ARAMIS trial revealed that the rate of favourable neurologic function at 90 days was 93.8% among patients assigned to dual antiplatelet therapy (DAPT) compared to those assigned to intravenous alteplase at 91.4%.
2. This study shows evidence that DAPT is noninferior (with a difference greater than the prespecified margin of -4.5%) to alteplase for patients with minor nondisabling acute ischemic stroke (AIS) within 4.5 hours of symptom onset.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Intravenous alteplase, a thrombolytic, is the current recommended management for patients with AIS who present within 4.5 hours of stroke onset. Minor strokes have been shown in previous studies to be effectively and safely managed by DAPT. The Antiplatelet vs R-tPA for Acute Mild Ischemic Stroke (ARAMIS) study sought to investigate whether intravenous alteplase should be administered for minor nondisabling stroke compared to DAPT. This randomized control trial found that in patients experiencing a nondisabling minor stroke within 4.5 hours, short-term DAPT demonstrated comparable effectiveness to intravenous alteplase in terms of functional outcome at 90 days while carrying a lower risk of bleeding and less early neurological deterioration. One limitation is the lack of diversity in the patient population. Considering the differences in ischemic stroke characteristics among various populations, it may be important to seek additional confirmation of the study’s findings in other regions. This is necessary to determine the generalizability of the results to other populations and regions. In conclusion, the ARAMIS trial provides valuable input on the management of patients experiencing minor nondisabling AIS.
In-Depth [randomized controlled trial]: This study was a multicenter, open-label, blinded endpoint, noninferiority randomized clinical trial conducted between October 2018 and April 2022 at 38 hospitals in China. The 760 eligible participants of this study were adults who had experienced a minor AIS and were able to start receiving the study treatment within 4.5 hours of symptom onset. Exclusion criteria included pre-stroke disability, history of intracerebral hemorrhage, or definite indication for anticoagulation.
Patients were randomly assigned to receive either alteplase or DAPT. In the alteplase group, patients received guidelines-based treatment with alteplase, up to a maximum dose of 90mg. They also started guideline-based antiplatelet treatment 24 hours after receiving intravenous thrombolysis. In the DAPT group, patients received a loading dose of 300mg of clopidogrel on the first day, followed by a daily dose of 75mg for 12 (+2) days. They were also given 100mg of aspirin on the first day, followed by a daily dose of 100mg for 12 (+2) days. The choice of single antiplatelet therapy or DAPT for these patients was based on guidelines and continued for 90 days.
A Modified Rankin Score of 0 to 1 was the primary outcome which meant an excellent functional outcome at 90 days. The percentage of patients with favourable neurologic function at 90 days was 93.8% among patients assigned to DAPT, which was shown to be noninferior to intravenous alteplase at 91.4% (unadjusted 95% CI, −1.5%to 6.2%; P < .001 for noninferiority; adjusted 95% CI, −1.6% to 6.1%). Fewer patients in the DAPT group experienced early neurological deterioration at 24 hours (adjusted risk difference −4.6% [95% CI, −8.3% to −0.9%]) and a decreased risk of bleeding compared to the alteplase group.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.