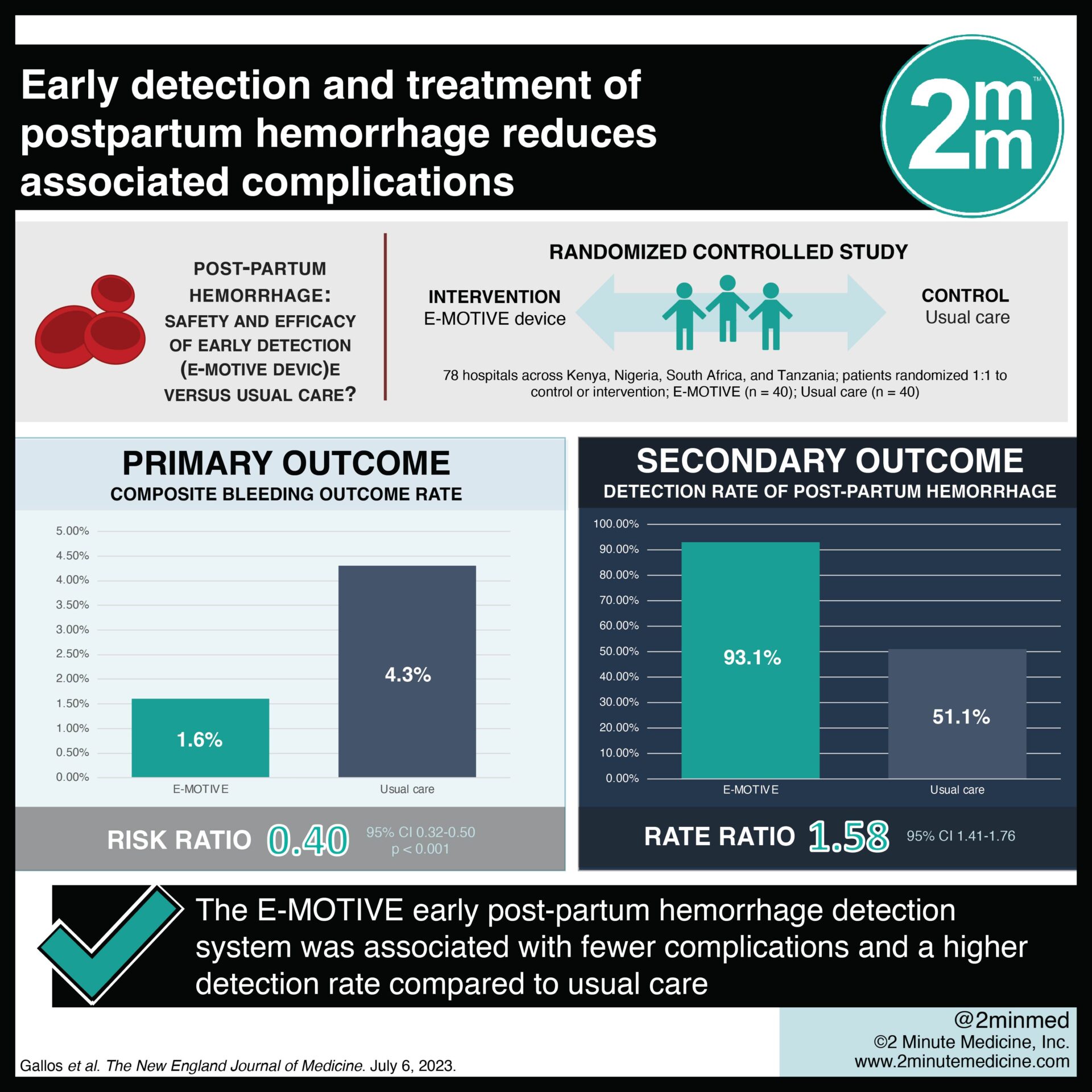
1. In this randomized controlled trial, the E-MOTIVE bundle, consisting of early detection and treatment of postpartum hemorrhage, was associated with a lower risk of complications than usual care.
2. The E-MOTIVE treatment bundle also resulted in higher detection rates of postpartum hemorrhage and treatment adherence compared to usual care.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The adoption of the World Health Organization (WHO) recommendations for the prevention and treatment of postpartum hemorrhage is poor. This is attributed to poor detection rates, inconsistent interventions, and local challenges such as limited staffing, lack of relevant knowledge, and low engagement. In this clinical trial, administratively distinct hospitals in Kenya, Nigeria, South Africa, and Tanzania were randomized in a 1:1 ratio to either the E-MOTIVE intervention or usual care control. The E-MOTIVE intervention is made up of a calibrated drape for postpartum hemorrhage detection and a standardized WHO-recommended treatment package. The primary outcome of a composite of severe postpartum hemorrhage, postpartum laparotomy for bleeding, or maternal death from bleeding occurred at a significantly lower rate in the intervention group than in the control group. All three individual components of the primary outcome occurred less frequently in the intervention group. Identification of postpartum hemorrhage and treatment adherence were higher in the intervention group. For limitations, clinical outcomes, such as postnatal hemoglobin level and anemia, and patient experience were not collected. The trial was also not powered to assess maternal death.
In-Depth [randomized controlled trial]: In this clinical trial, the E-MOTIVE intervention for postpartum hemorrhage was assessed in administratively distinct hospitals in Kenya, Nigeria, South Africa, and Tanzania. The E-MOTIVE intervention is a first-aid package made up of a calibrated device for postpartum hemorrhage detection, along with a combination of WHO-recommended treatments that are immediately available. The analysis consisted of 78 hospitals randomized into the E-MOTIVE intervention group (n=40; patient sample size n=49,101) or control group (n=40; patient sample size n=50,558). The primary outcome of a composite of severe postpartum hemorrhage, postpartum laparotomy for bleeding, or maternal death from bleeding occurred at a significantly lower rate in the intervention group (1.6%) than in the control group (4.3%) (risk ratio, 0.40; 95% Confidence Interval [CI], 0.32 to 0.50; p<0.001). The detection rate of postpartum hemorrhage was 93.1% in the intervention group and 51.1% in the control group (rate ratio, 1.58; 95% CI, 1.41 to 1.76). The adherence rate was 91.2% in the intervention group and 19.4% in the control group (rate ratio, 4.94; 95% CI, 3.88 to 6.28). In the intervention and control groups, postpartum hemorrhage was diagnosed in 8.5% and 16.7%, respectively (risk ratio, 0.51; 95% CI, 0.44 to 0.60), and severe postpartum hemorrhage in 1.6% and 4.3%, respectively (risk ratio, 0.39; 95% CI, 0.31 to 0.49). In summary, the E-MOTIVE intervention improves the detection of postpartum hemorrhage, reduces postpartum hemorrhage complications, and improves treatment adherence.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.