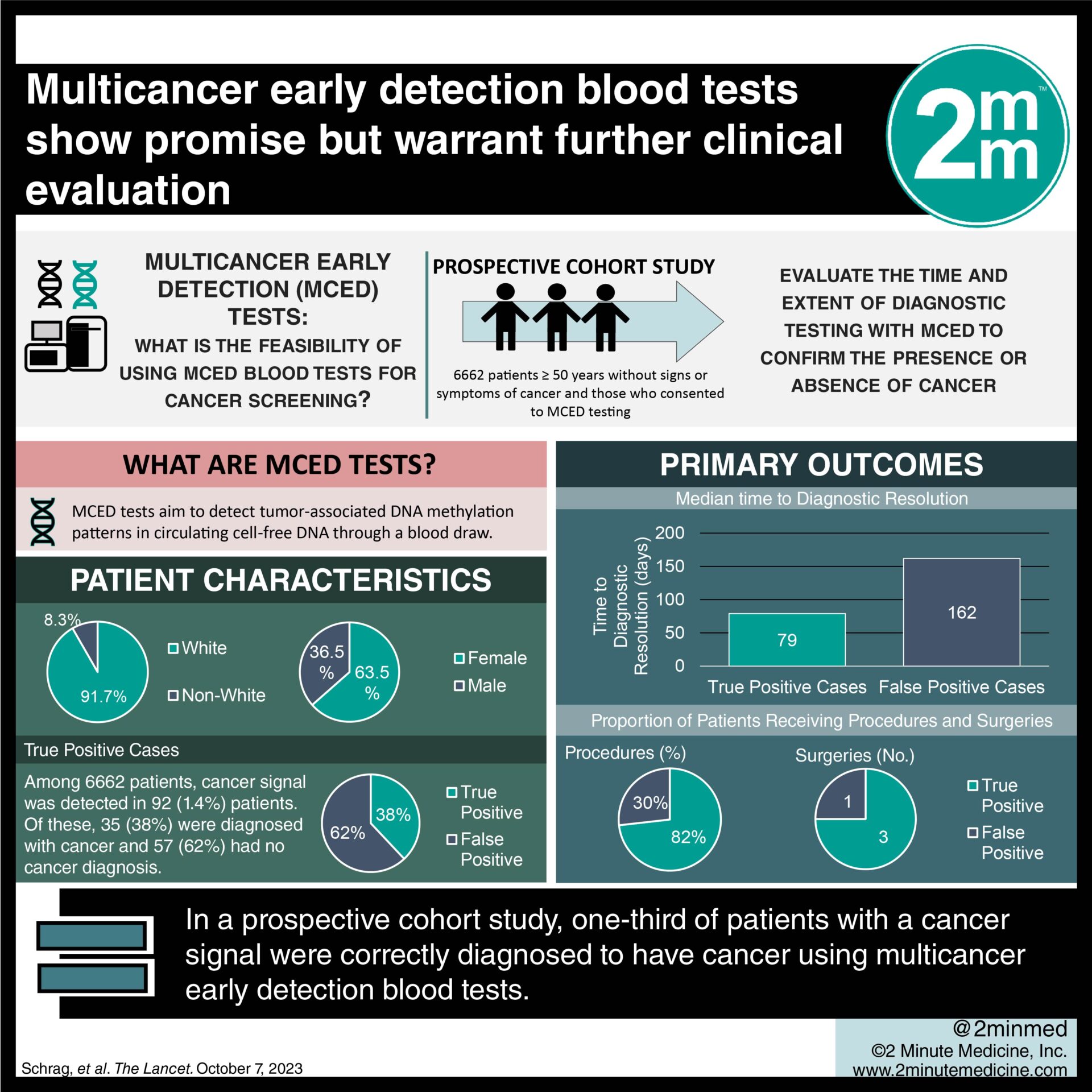
1. One-third of patients with a cancer signal were correctly diagnosed to have cancer.
2. Patients in the true-positive group underwent more procedures and surgeries compared to those in the false-positive group.
Evidence Rating Level: 2 (Good)
Study Rundown: Cancer is a leading cause of death worldwide with nearly two million new cases expected to be diagnosed this year. Early cancer screening, particularly for breast, lung, colorectal, and cervical cancers, may reduce cancer-related mortality. The multicancer early detection (MCED) tests aim to detect tumor-associated DNA methylation patterns in cell-free DNA through an easy-to-obtain blood draw. MCED blood tests have the potential to improve screening efficacy and accuracy, but the evidence is currently limited. This prospective cohort study aimed to evaluate the time and extent of diagnostic testing with MCED to confirm the presence or absence of cancer. The primary outcome of this study was time to diagnostic resolution, marked as either a true positive or false positive. According to study results, approximately one-third of all patients had a correct diagnosis of cancer while the remaining two-thirds received false-positive results. Although this study was well done, it was limited by an overdiagnosis of cancer as well as the need for further research to determine the clinical utility of MCED screening. Furthermore, the cohort consisted primarily of Caucasian participants which would limit its generalizability.
Click to read the study in The Lancet
Relevant Reading: Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death
In-depth [prospective cohort]: Between Dec 12, 2019, and Dec 4, 2020, 6662 patients were enrolled from 7 different US health networks. Included were patients ≥ 50 years without signs or symptoms of cancer and those who consented to MCED testing. Altogether, 6621 patients were included in the final analysis. The majority of patients were White (91.7%) and female (63.5%). The primary outcome of median time to diagnostic resolution was 79 days (interquartile range [IQR] 37-219), with 57 days (IQR 33-143) for true-positive cases and 162 days (IQR 44-248) for false-positive cases. Among patients in which a cancer signal was detected (92 of 6621, 1.4%), 38% were diagnosed with cancer (true positives) while 62% had no cancer diagnosis (false positives). Compared to the false-positive group, patients in the true-positive group had more procedures (82% vs. 30%) and surgeries (3 in true-positive vs. 1 in false-positive). Findings from this study suggest that MCED screening for cancer is feasible, emphasizing the need for further research to assess its clinical utility.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.