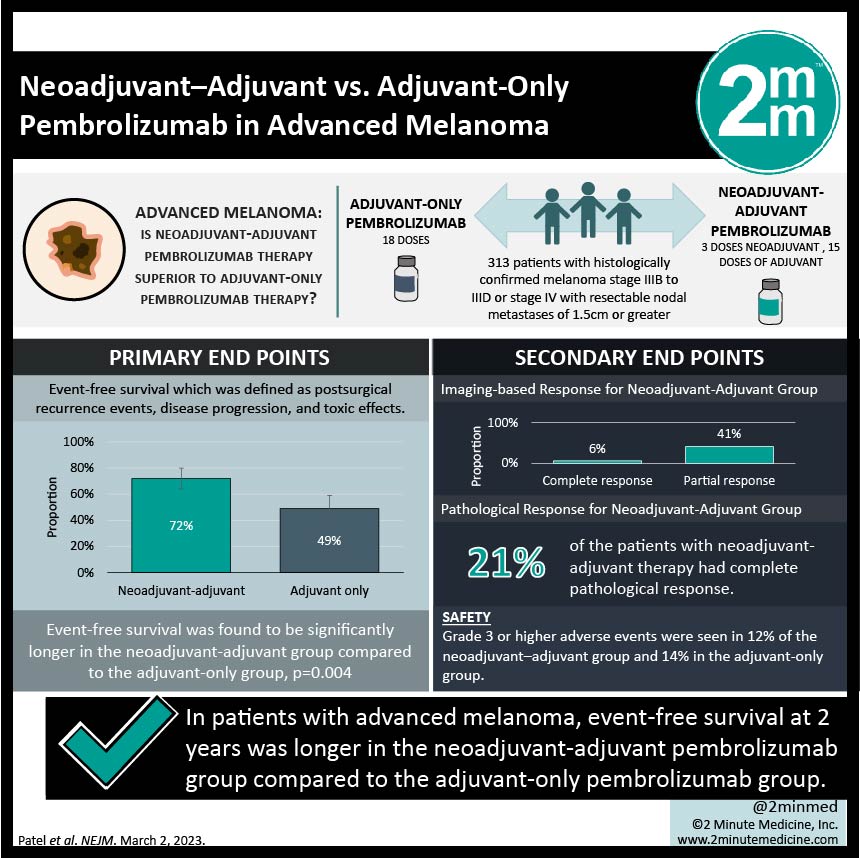
2. Grade 3 or higher adverse events were seen at similar rates across both groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Patients with advanced melanoma (stage III or IV) are at high risk of relapse after undergoing surgical excision. Administering anti-PD-1 therapy has been shown to improve outcomes in the adjuvant setting but recent research has demonstrated benefit in the neoadjuvant setting due to the potential of activating antitumor T cells that are in the bulk of the tumour. This phase 2 study investigated this with the primary endpoint being event-free survival which was defined as postsurgical recurrence events, disease progression, and toxic effects. It was found that at 2 years, event-free survival was 72% in the neoadjuvant-adjuvant group vs 49% in the adjuvant-only group with overall event-free survival being significantly longer in the neoadjuvant-adjuvant group compared to the adjuvant-only group, p=0.004. This benefit of neoadjuvant therapy was seen across all subgroups. After neoadjuvant therapy, there was both an imaging-based response (6% complete, 41% partial) and a pathological response (21% complete). Grade 3 or higher adverse events were seen in 12% of the neoadjuvant–adjuvant group and 14% in the adjuvant-only group. The strength of this study was the length of follow-up. The limitations of this study included the small sample size and the composite endpoint. Overall, this study showed that neoadjuvant followed by adjuvant immunotherapy had longer event-free survival than adjuvant therapy alone in patients with stage III-IV melanoma and warrants further study to determine the more optimal curative strategy.
Click to read the study in NEJM
Relevant Reading: Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease.
In-Depth [randomized controlled trial]: This open-label phase 2 randomized control trial recruited patients with histologically confirmed melanoma stage IIIB to IIID or stage IV with resectable nodal metastases of 1.5cm or greater. Patients were randomly assigned to receive 3 doses of pembrolizumab as neoadjuvant therapy followed by 15 doses as adjuvant therapy (154 patients) vs receiving 18 doses as adjuvant therapy (159). The median follow-up time was 14.7 months. At 2 years, event-free survival was 72% (95%CI, 64-80) in the neoadjuvant-adjuvant group vs 49% (95%CI, 41-59) in the adjuvant-only group. Overall, event-free survival was found to be significantly longer in the neoadjuvant-adjuvant group compared to the adjuvant-only group, p=0.004. The benefit of neoadjuvant therapy was seen across all subgroups. Initial response to neoadjuvant therapy was investigated and it was found that 6% of patients had a complete imaging-based response, 41% had a partial imaging response, and 21% had a complete pathological response. Grade 3 or higher adverse events were seen in 12% of the neoadjuvant–adjuvant group and 14% in the adjuvant-only group. There were no deaths attributed to pembrolizumab in either group. Overall, this study showed that neoadjuvant-adjuvant therapy with pembrolizumab had longer event-free survival than the standard-care adjuvant pembrolizumab alone in patients with stage III-IV melanoma.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.












