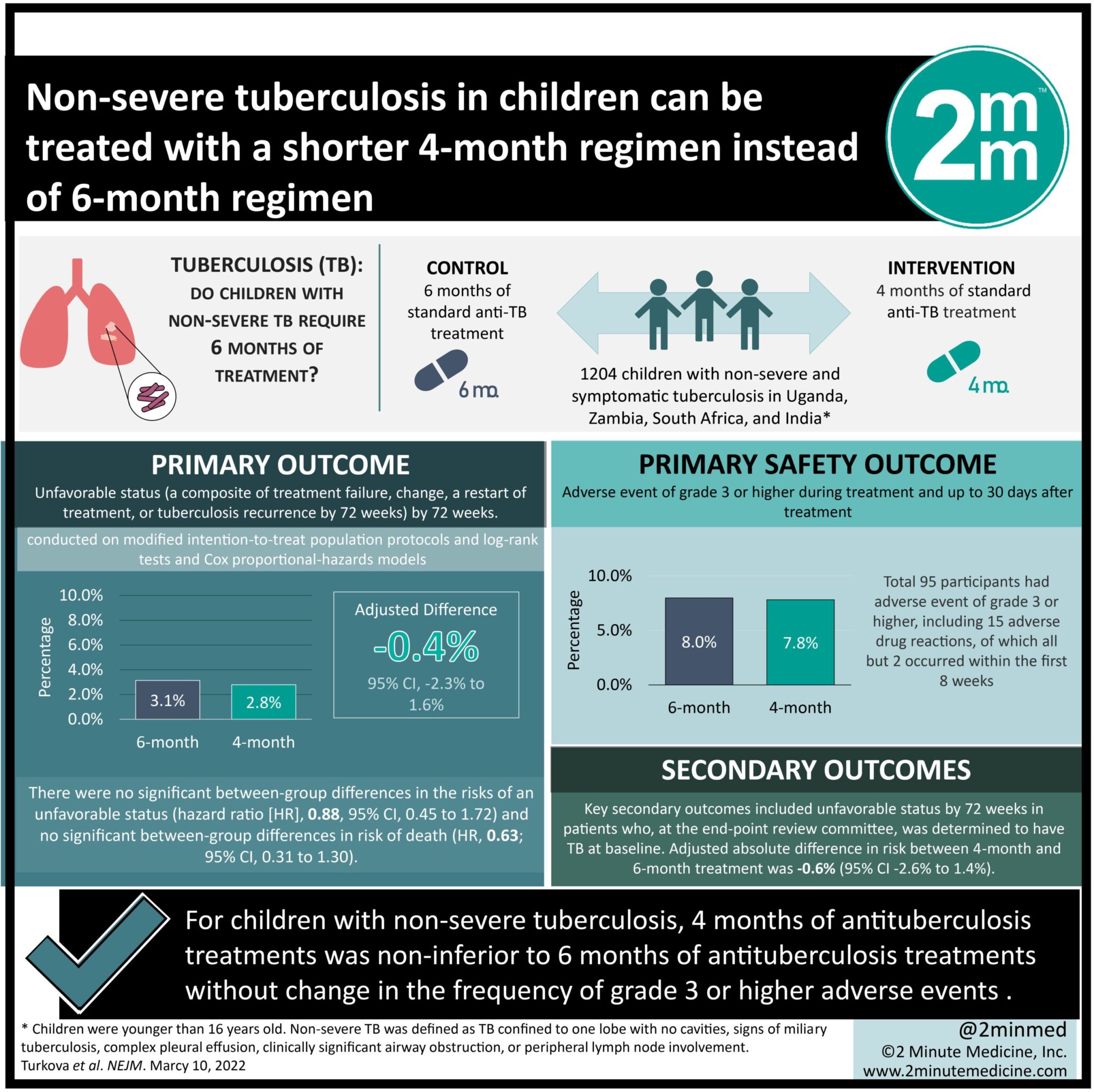
- For children with non-severe tuberculosis, 4 months of antituberculosis treatments was non-inferior to 6 months of antituberculosis treatments.
- For children with non-severe tuberculosis, a shorter treatment regimen did not significantly change the frequency of adverse events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Tuberculosis affects more than 1 million children annually with a mortality rate of about 20%. However, children typically have non-severe, smear-negative tuberculosis compared to adults, but current guidelines recommend 6 months of antituberculosis treatment in children, which is the same length of time as treatment in adults. Therefore, there is a gap in knowledge as to understanding whether 4 months of antituberculosis treatment would be as effective as 6 months of treatment in children with non-severe, smear-negative, drug-susceptible tuberculosis. This study found that 4 months of antituberculosis treatment was non-inferior to 6 months of antituberculosis treatment in children with non-severe tuberculosis. This study was limited by being an open-label trial which can lead to more frequent treatment extensions in the four-month group, as well as the generalizability of the results to settings where chest radiographs are not available for characterizing non-severe tuberculosis. Nevertheless, these study’s findings are significant, as they demonstrate that 4 months of antituberculosis treatment is effective for treating children with non-severe, drug-susceptible, smear-negative tuberculosis.
Click to read the study in NEJM
Relevant Reading: Childhood Tuberculosis — Time for Shorter and Differentiated Treatment
In-Depth [randomized control trial]: This open-label, treatment-shortening, noninferiority trial studied 1204 children with non-severe and symptomatic tuberculosis in Uganda, Zambia, South Africa, and India from July 2016 to July 2018. Patients who were younger than 16 years of age who had symptomatic non-severe and smear-negative tuberculosis were included in the study. Non-severe tuberculosis was characterized as respiratory tuberculosis confined to one lobe with no cavities, no signs of miliary tuberculosis, no complex pleural effusion, and no clinically significant airway obstruction. Patients who did not fit these criteria were excluded from the study. Patients were either assigned to receive 4 months of antituberculosis treatment or the standard 6 months of treatment using WHO-recommended pediatric doses. The primary outcome was unfavorable status, a composite of treatment failure, change, a restart of treatment, or tuberculosis recurrence by 72 weeks. The primary safety outcome was an adverse event of grade 3 or higher during treatment and up to 30 days after treatment. Outcomes in the primary analysis were conducted on modified intention-to-treat population protocols and log-rank tests and Cox proportional-hazards models. Based on the analysis, the noninferiority of 4 months of antituberculosis treatment was consistent across the intention-to-treat, per-protocol, and key secondary analyses. An unfavorable outcome was reported in 3% of the 4-month group and 3% in the 6-month group (95% confidence interval [CI], -2.3 to 1.6). There were no significant between-group differences in the risks of an unfavorable status (hazard ratio [HR], 0.88; 95% CI, 0.45 to 1.72) and no significant between-group differences in risk of death (HR, 0.63; 95% CI, 0.31 to 1.30). Moreover, 8% of participants had an adverse event of grade 3 or higher, all but 2 of which occurred within the first 8 weeks when the treatments were the same in the two groups. Overall, this study demonstrated 4 months of antituberculosis treatment was non-inferior to 6 months of antituberculosis treatment in children with non-severe tuberculosis in Africa and India. This encourages a stratified therapeutic approach to drug-susceptible non-severe tuberculosis in children.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.