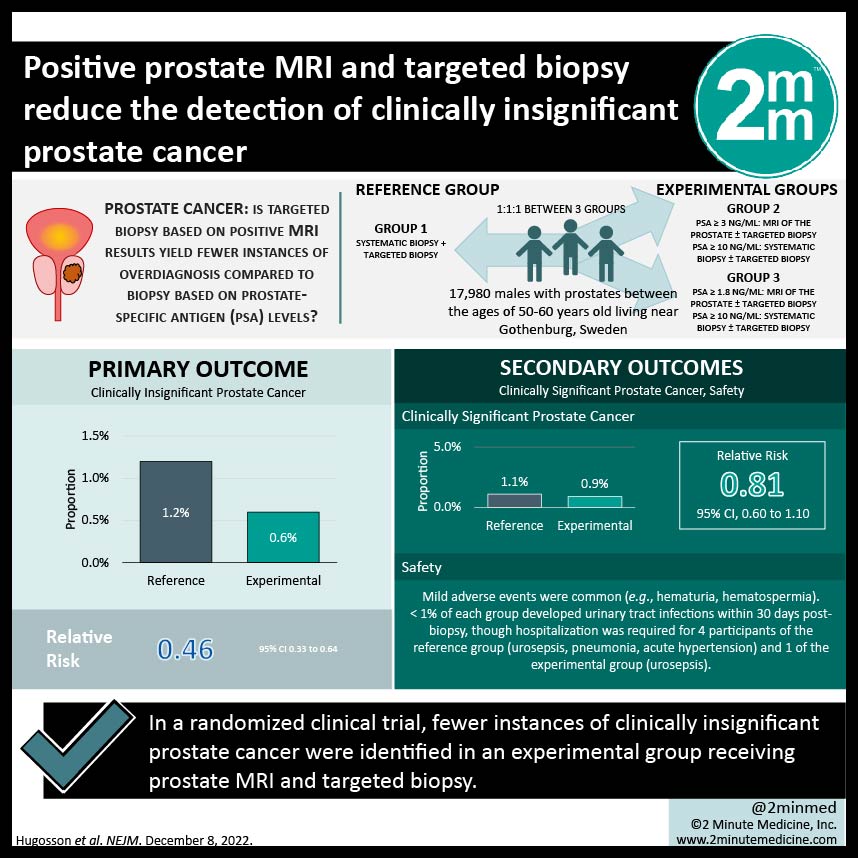
1. Fewer instances of clinically insignificant prostate cancer were identified in an experimental group receiving prostate MRI and targeted biopsy
2. Severe adverse events rare in both groups
Evidence Rating Level: 1 (Excellent)
Study Rundown: Current methods for population-based prostate cancer screening is associated with high rates of overdiagnosis. This study investigated whether targeted biopsy based on positive MRI results would yield fewer instances of overdiagnosis, as compared to systematic biopsy for all males with elevated PSA levels. The primary outcome of this study was clinically insignificant prostate cancer, while the secondary outcome was clinically significant prostate cancer and safety. There were statistically significantly fewer instances of clinically insignificant prostate cancer identified in the experimental group compared to the reference group, while the instances of clinically significant prostate cancer identified in each group were similar. However, 2% of the reference group had cancers only detected via systemic biopsy, all of which were deemed lower-volume diseases and managed with surveillance. Only a few adverse events (AEs) occurred in both groups, with hematuria and hematospermia being the most common. Limitations to this study include the restriction of participation to males between the ages of 50 and 60, as well as conducting the study out of a single centre. As a result, conclusions should be interpreted and applied cautiously. Overall, the results from this study suggest that modifying prostate cancer screening to include prostate MRI before biopsy of patients with elevated PSA levels, and performing a targeted biopsy of lesions with specific severity parameters minimizes the risk of identifying clinically insignificant prostate cancers by almost half but with the trade-off of missing a small proportion of missing initially lower volume disease.
Click to read the study in NEJM
Relevant Reading: Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer MortalityThe CAP Randomized Clinical Trial
In-Depth [randomized controlled trial]: This randomized controlled trial enrolled 17,980 males with prostates between the ages of 50-60 years old living near Gothenburg, Sweden. They were randomly allocated in a 1:1:1 manner to the reference group (n=5,994) or experimental group (n= 11, 986) which was split into two groups (trial groups 2 and 3). Individuals in group 2 with PSA levels ≥ 3ng/mL underwent an MRI of the prostate ± targeted biopsy. For participants with PSA ≥ 10ng/mL, systematic biopsy ± targeted biopsy was completed regardless of MRI findings. In group 3, methods remained the same as in group 2 but used a reduced cut-off for PSA levels at ≥ 1.8ng/mL. This study only reports results from participants with PSA levels greater than 3 ng/mL. Clinically insignificant prostate cancer was identified in 0.6% of the experimental group and 1.2% of the reference group (relative risk (RR), 0.46; 95% confidence interval (CI), 0.33 to 0.64). In the experimental group, clinically significant prostate cancer was identified in 0.9% of the participants, as compared to 1.1% of reference group participants (RR, 0.81; 95% CI, 0.60 to 1.10). In both the reference and experimental groups, mild adverse events were common and included hematuria and hematospermia. Less than 1% of each group developed urinary tract infections within 30 days post-biopsy, though hospitalization was required for 4 participants of the reference group (urosepsis, pneumonia, acute hypertension) and 1 of the experimental group (urosepsis). There were no deaths recorded in 30 days post-biopsy.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.