2. The RSVpreF vaccine also was associated with significantly decreased rates of acute respiratory illness in adults aged 60+.
Evidence Rating Level: 1 (Excellent)
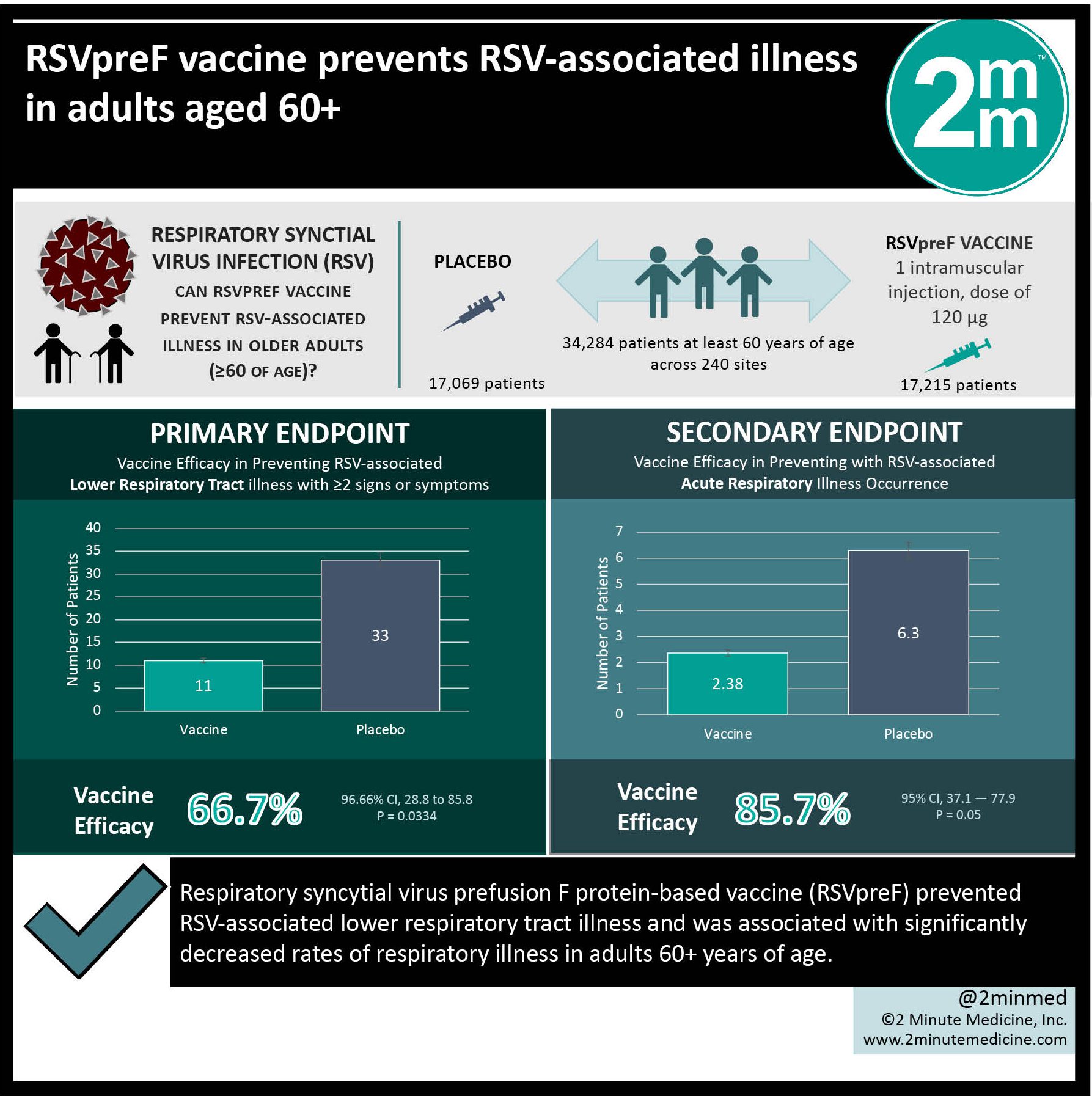
Study Rundown: RSV is a common cause of lower respiratory tract illness worldwide, with severe illness being most prevalent in young children and older adults. The severity of illness among older adults who are hospitalized with RSV disease is substantial; 18% are admitted to an intensive care unit, 31% receive home health services at discharge, and 26% die within one year after admission. However, an RSV vaccine has not yet been developed, though the investigational bivalent RSVpreF vaccine has recently been developed to target the RSV fusion glycoprotein. There is still a gap in knowledge as to understanding the efficacy and safety of the RSVpreF vaccine in the first RSV season after injection. Overall, this study found that the RSVpreF vaccine had an acceptable safety profile and prevented RSV-associated respiratory illness in adults greater than 60 years of age. This study was limited by the exclusion of immunocompromised persons and was only evaluated during a single RSV season. Nevertheless, these study’s findings are significant, as they demonstrate that the RSVpreF vaccine is safe and effective in preventing RSV-associated respiratory illness in older adults.
Click to read the study in NEJM
Relevant Reading: Efficacy and Safety of an Ad26.RSV.preF–RSV preF Protein Vaccine in Older Adults
In-Depth [randomized controlled trial]: This double-blinded, randomized, placebo-controlled trial was conducted to study the efficacy and safety of the RSVpreF vaccine in older adults. Patients at least 60 years of age were eligible for the study, including those with chronic conditions, including chronic cardiopulmonary disease (e.g., chronic obstructive pulmonary disease and asthma), and were recruited from 240 sites. Patients who were immunocompromised were excluded from the study. The primary outcome measured was the efficacy of the RSVpreF vaccine in preventing RSV-associated lower respiratory tract illness with at least two signs or symptoms lasting more than one day and RSV infection that was confirmed using reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay within seven days after the onset of signs or symptoms. Outcomes in the primary analysis were assessed via conditional exact test based on the binomial distribution of the number of cases in the RSVpreF vaccine group. Based on the primary analysis, RSV-associated lower respiratory tract illness with at least two signs or symptoms occurred in 11 participants in the vaccine group (1.19 cases per 1,000 person-years of observation) and 33 participants in the placebo group (3.58 cases per 1,000 person-years of observation). Vaccine efficacy was 66.7% (96.66% Confidence Interval [CI], 28.8 to 85.8). RSV-associated acute respiratory illness occurred in 22 participants in the vaccine group (2.38 cases per 1,000 person-years of observation) and 58 participants in the placebo group (6.30 cases per 1000 person-years of observation) with vaccine efficacy of 62.1% (95% CI, 37.1 to 77.9). In summary, this study demonstrates the safety and efficacy of the RSVpreF vaccine in older adults.














