2. There were significantly more adverse events in the active tDCS group.
Evidence Rating Level: 1 (Excellent)
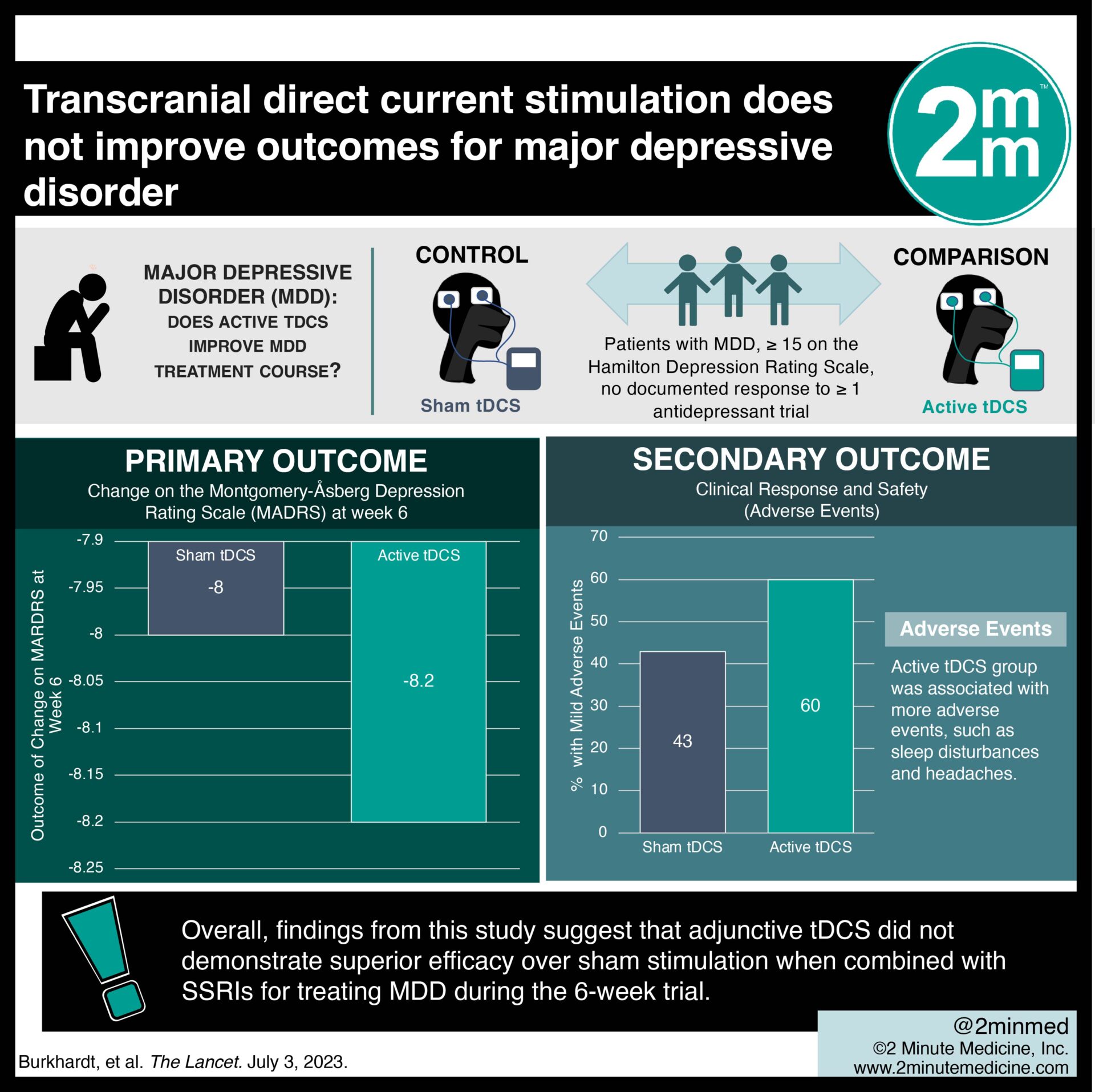
Study Rundown: Transcranial direct current stimulation (tDCS) has been proposed as a feasible treatment for major depressive disorder (MDD), but heterogeneous meta-analytic evidence and limited multicentre trial data exist. This randomized controlled trial aimed to assess the efficacy of active tDCS as an add-on treatment to a stable dose of selective serotonin reuptake inhibitors (SSRIs) in adults with MDD. The primary outcome of this study was change on the Montgomery-Åsberg Depression Rating Scale (MADRS) at week 6, while key secondary outcomes were clinical response and safety. According to study results, there was no significant difference in MADRS score between active and sham tDCS groups. In addition, the tDCS group was associated with more adverse events, such as sleep disturbances and headaches. Although this study was well done, it was limited by a relatively short 6-week assessment period that may not have been sufficient to measure the effects.
Click to read the study in The Lancet
Relevant Reading: Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression
In-depth [randomized-controlled trial]: Between Jan 19, 2016, and Jun 15, 2020, 3601 patients were screened for eligibility across 8 hospitals in Germany. Included were patients ≥ 18 years with MDD, a score ≥ 15 on the Hamilton Depression Rating Scale, and no documented response to ≥ 1 antidepressant trial. Altogether, 150 patients (77 in active tDCS and 73 in sham tDCS) were included in the final analysis. The primary outcome of change on MADRS at week 6 was comparable between the active tDCS group (-8.2, standard deviation [SD] 7.2) and the sham tDCS group (-8.0, SD 9.3). Notably, significantly more mild adverse events occurred in the active tDCS group (60%) compared to the sham tDCS group (43%, p=0.028). Overall, findings from this study suggest that adjunctive tDCS did not demonstrate superior efficacy over sham stimulation when combined with SSRIs for treating MDD during the 6-week trial.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.














