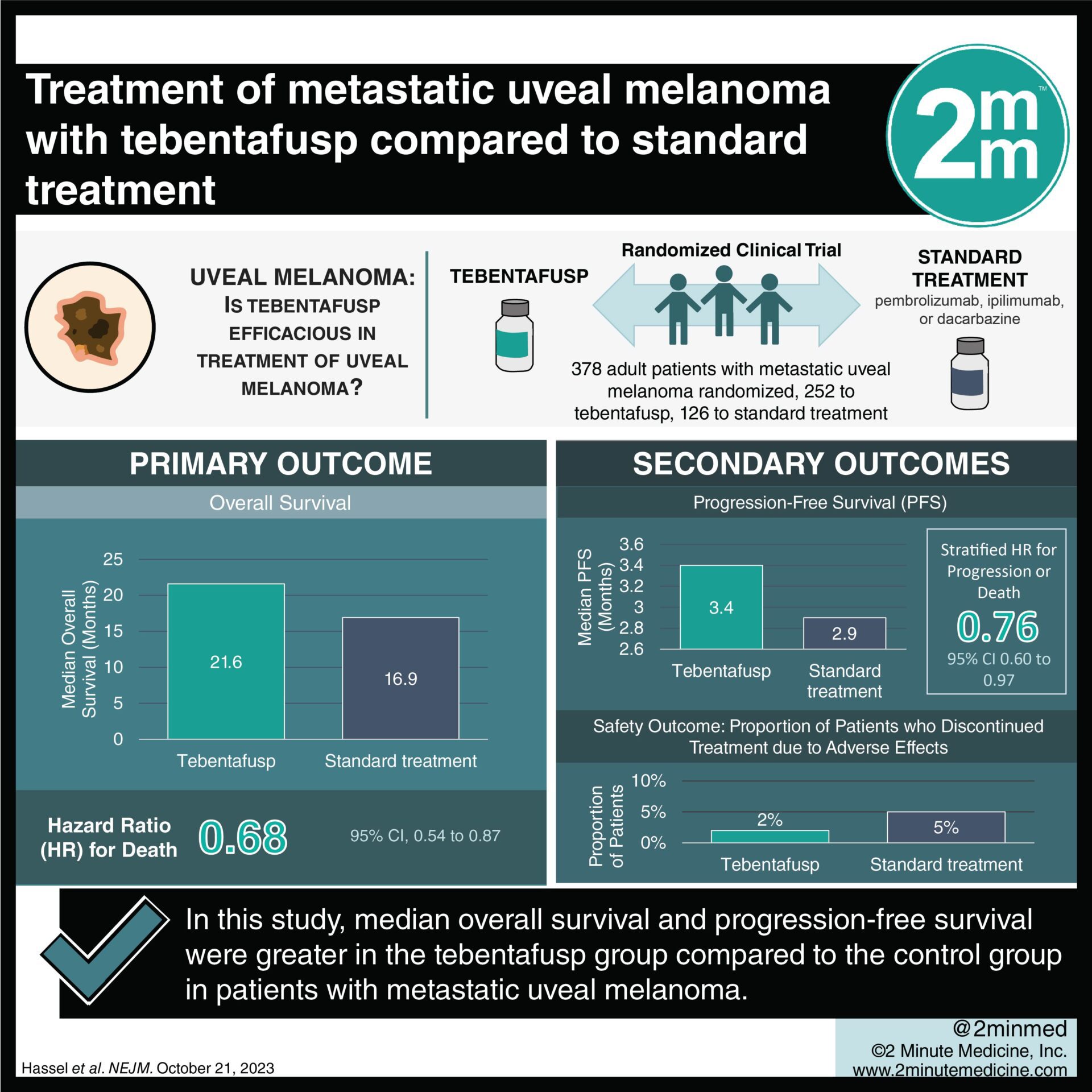
1. Median overall survival and progression-free survival were greater in the tebentafusp group compared to the control group.
2. Adverse events were generally similar between both groups, with rash the most common presentation.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Almost 50% of patients who have uveal melanoma are affected by metastatic disease, which is associated with an overall survival of roughly 12 months. While immune checkpoint inhibitors have been effective in the treatment of cutaneous melanoma, they have not shown such outcomes in treating uveal melanoma. In previous phase 1 and 2 studies tebentafusp, a T-cell receptor-bispecific fusion (BiTEs) protein, has shown survival outcomes almost double that of historical outcomes. This phase 3 study examined the effect on survival outcomes of tebentafusp in patients with metastatic uveal melanoma as compared to standard treatment. The primary outcome of this study was the overall survival (OS). Secondary outcomes included progression-free survival (PFS) as well as safety outcomes. Median OS was greater in the test group who received tebentafusp when compared with the control group (21.6 months vs. 16.9 months). Median PFS was greater in the test group (3.4 months) compared to the control group (2.9 months). Additionally, there were more patients in the test group who saw any amount of tumour size reduction (40% vs. 24%). Adverse events (AEs) were similar in both the test and control groups with rash comprising the most common treatment-related AE of any severity (83% and 27%, respectively). Limitations of this study include that there were only three treatment types included in the control group, limiting the extrapolation of the data to other standard treatment options. Overall, the results from this study support that treatment with tebentafusp provides greater survival outcomes as compared to standard treatment options.
Click to read the study in The New England Journal of Medicine
Relevant Reading: Clinical and molecular response to tebentafusp in previously treated patients with metastatic uveal melanoma: a phase 2 trial
In-Depth [randomized controlled trial]: This phase 3 randomized controlled trial analyzed data from multiple countries. 378 adult patients with metastatic uveal melanoma were included and randomized in a 2:1 manner to receive either tebentafusp (test group; n = 252) or standard treatment – pembrolizumab, ipilimumab, or dacarbazine (control group; n = 126). 245 patients in the tebentafusp and 111 in the control group received treatment during the study trial. Patients from this study were followed for a minimum of 3 years, with a median follow-up of 43.3 months. The median OS in the tebentafusp group was 21.6 months (95% confidence interval (CI), 19.0 to 24.3 months) while the median OS in the test group was 16.9 months (95% CI, 12.9 to 19.5). Median PFS in the test group was 3.4 months (95% CI, 3.0- 5.4) compared to 2.9 months in the control group (95% CI, 2.8-to 3.0 months) in the control group (stratified hazard ratio (HR) for progression or death, 0.76; 95% CI, 0.60-0.97). Only 2% of tebentafusp test group patients discontinued treatment, as compared to 5% in the control group and there were no deaths during the study related to treatments.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.