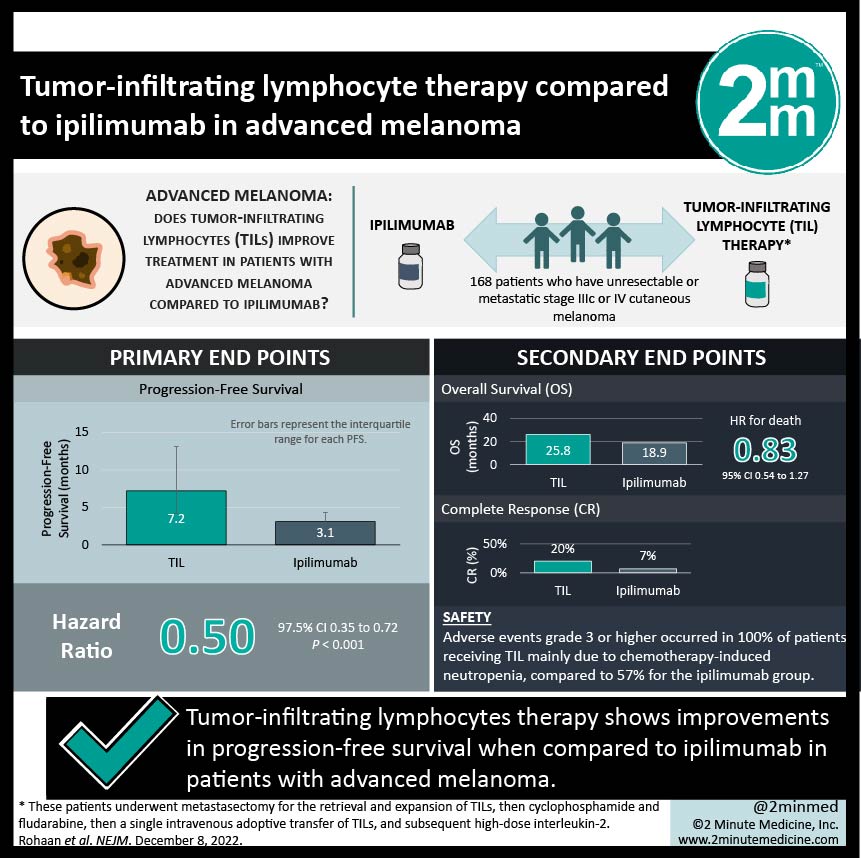
1 .Tumor-infiltrating lymphocytes therapy shows improvements in PFS when compared to ipilimumab
2. All patients with tumor-infiltrating lymphocytes therapy had grade 3 or higher adverse events, mainly related to chemotherapy-related neutropenia.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Patients with metastatic melanoma have various treatment options available such as PD-1 inhibitors, anti–cytotoxic T-lymphocyte antigen 4 (CTLA-4), and BRAF and MEK inhibition, but these do not confer durable benefits to all patients. Adoptive cell therapy with tumor-infiltrating lymphocytes (TILs) is an autologous treatment that involves the outgrowth of tumor-resident T cells with interleukin-2 to enhance the expansion of the cells and augment antitumor responses. This is a phase 3 study that explores TILs vs ipilimumab as first or second-line treatment in patients with advanced melanoma. The primary endpoint was progression-free survival (PFS), and secondary endpoints included overall survival (OS), complete response (CR), health-related quality of life (HRQoL), and safety. This study found improvement in PFS (7.2 months in the TIL group vs 3.1 months with ipilimumab, HR 0.50), OS (25.8 months in the TIL group vs 18.9 months in the ipilimumab group, HR 0.83 [not significant]), and CR (20% in the TIL group vs 7% in the ipilimumab group). Safety was generally worse in the TIL group as adverse events grade 3 or higher occurred in 100% of patients mainly due to chemotherapy-induced neutropenia, compared to 57% for the ipilimumab group. HRQoL was slightly higher in the TIL group vs the ipilimumab group with regards to global QoL, physical functioning, and emotional functioning. The strengths of this study included capturing a wide variety of outcome measures. The limitations of this study include the small sample size and the non-blinded nature of the treatments. Overall, this study shows that in patients with advanced melanoma, TILs therapy shows promising results when compared to immune-checkpoint blockade and further studies will clarify the role in advanced melanoma treatment.
Click to read the study in NEJM
Relevant Reading: Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma
In-Depth [randomized controlled trial]: This is a multicenter, open-label, phase 3 trial with patients who have unresectable or metastatic stage IIIc or IV cutaneous melanoma, that were randomized to receive either TILs (84 patients) or ipilimumab (84 patients). Those assigned to TIL underwent metastasectomy for the retrieval and expansion of TILs, followed by cyclophosphamide and fludarabine, then a single intravenous adoptive transfer of TILs, and subsequent high-dose interleukin-2. The overall median follow-up time was 33 months. PFS was 7.2 months (95%CI, 4.2 to 13.1) in the TIL group vs 3.1 months (95%CI, 3.0 to 4.3) with ipilimumab, HR 0.50 (95%CI, 0.35 to 0.72; P<0.001). Median OS in the TIL group was 25.8 months (95%CI, 18.2 to not reached) vs 18.9 months (95%CI, 13.8 to 32.6) in the ipilimumab group, HR 0.83 (95%CI, 0.54 to 1.27). Complete responses were seen in 20% (95%CI, 12 to 30) in the TIL group and 7% (95%CI, 3 to 15) in the ipilimumab group. Treatment-related adverse events grade 3 or higher occurred in 100% of the TIL group mainly due to chemotherapy-induced neutropenia but other adverse events included CR increase, hypertension, and hypoxia. For the ipilimumab group, treatment-related adverse events grade 3 or higher occurred in 57% of patients. HRQoL was overall higher in the TIL group vs the ipilimumab group, with global QoL mean scores 77.4 vs 69.6, physical functioning 82.0 vs 79.1, and emotional functioning 85.4 vs 75.7. Overall, this paper shows that treatment with TILs is promising compared to treatment with ipilimumab in patients with advanced melanoma.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.