1. In this randomized controlled trial, withholding prophylactic platelet transfusion in patients with thrombocytopenia was associated with worse outcomes following central venous catheter (CVC) placement.
2. Withholding prophylactic platelet transfusion in patients with thrombocytopenia resulted in an increased risk of CVC-related bleeding events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: CVC placement is a frequently performed invasive procedure. However, there is a lack of good-quality evidence as to standardized platelet transfusion protocols or validated bleeding assessment tools in patients with severe thrombocytopenia. Though, the reported bleeding risk after CVC placement is very low. Thus, a knowledge gap exists in understanding whether prophylactically transfused platelet concentrates are necessary to prevent CVC-related bleeding complications in patients with severe thrombocytopenia. Overall, this study found that withholding prophylactic platelet transfusion in patients with severe thrombocytopenia before CVC placement did not meet the predefined margin for noninferiority and resulted in more CVC-related bleeding than prophylactic platelet transfusion. This study was limited by being only conducted in the Netherlands and being a single-blind trial. Nevertheless, these study’s findings are significant, as they demonstrate that withholding prophylactic platelet transfusion is not as effective as platelet transfusion in preventing bleeding events in thrombocytopenic patients undergoing a CVC placement.
Click to read the study in NEJM
Relevant Reading: Ensuring a Reliable Platelet Supply in the United States
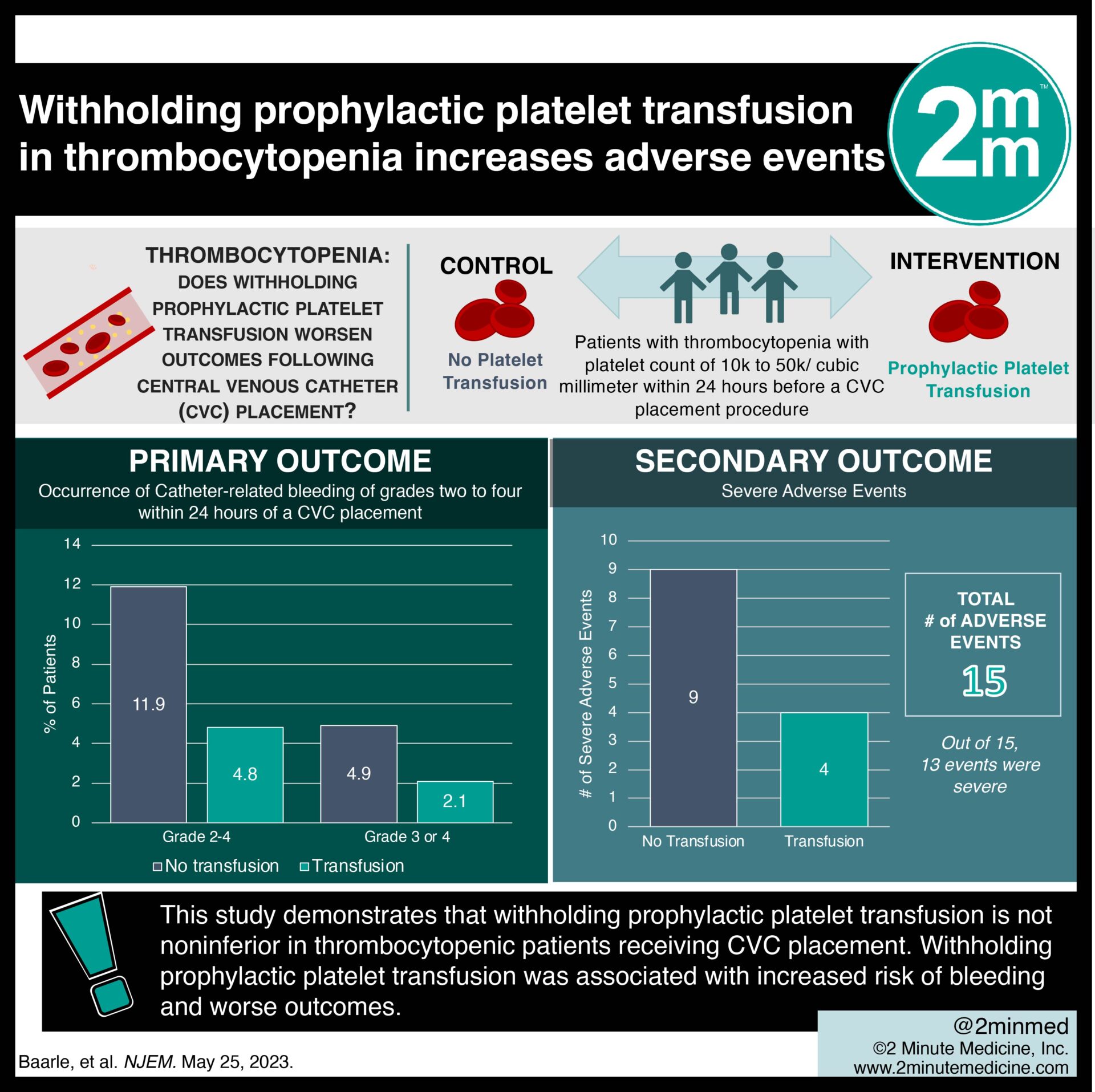
In-Depth [randomized controlled trial]: This randomized controlled trial was conducted across the Netherlands. Patients with thrombocytopenia who had a platelet count of 10,000 to 50,000 per cubic millimeter within 24 hours before a CVC placement procedure were eligible for the study. Patients who used a therapeutically administered anticoagulant, had a history of congenital or acquired coagulation factor deficiency, or a spontaneously prolonged international normalized ratio (INR) of 1.5 or more were excluded from the study. The primary outcome measured was the occurrence of catheter-related bleeding of grades two to four within 24 hours of a CVC placement. Outcomes in the primary analysis were assessed via relative risks with a Poisson mixed-effects model. Based on the primary analysis, catheter-related bleeding of grades two to four occurred in nine of 188 patients (4.8%) in the transfusion group and in 22 of 185 patients (11.9%) in the no-transfusion group (relative risk, 2.45; 90% confidence interval [CI], 1.27 to 4.70). Catheter-related bleeding of grades two to four occurred in four of 188 patients (2.1%) in the transfusion group and in nine of 185 patients (4.9%) in the no-transfusion group (relative risk, 2.43; 95% CI, 0.75 to 7.93). In summary, 15 adverse events were observed. Of these events, 13 (four in the transfusion group and nine in the no-transfusion group) were categorized as severe. This study demonstrates that withholding prophylactic platelet transfusion is not noninferior in thrombocytopenic patients receiving CVC placement.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.