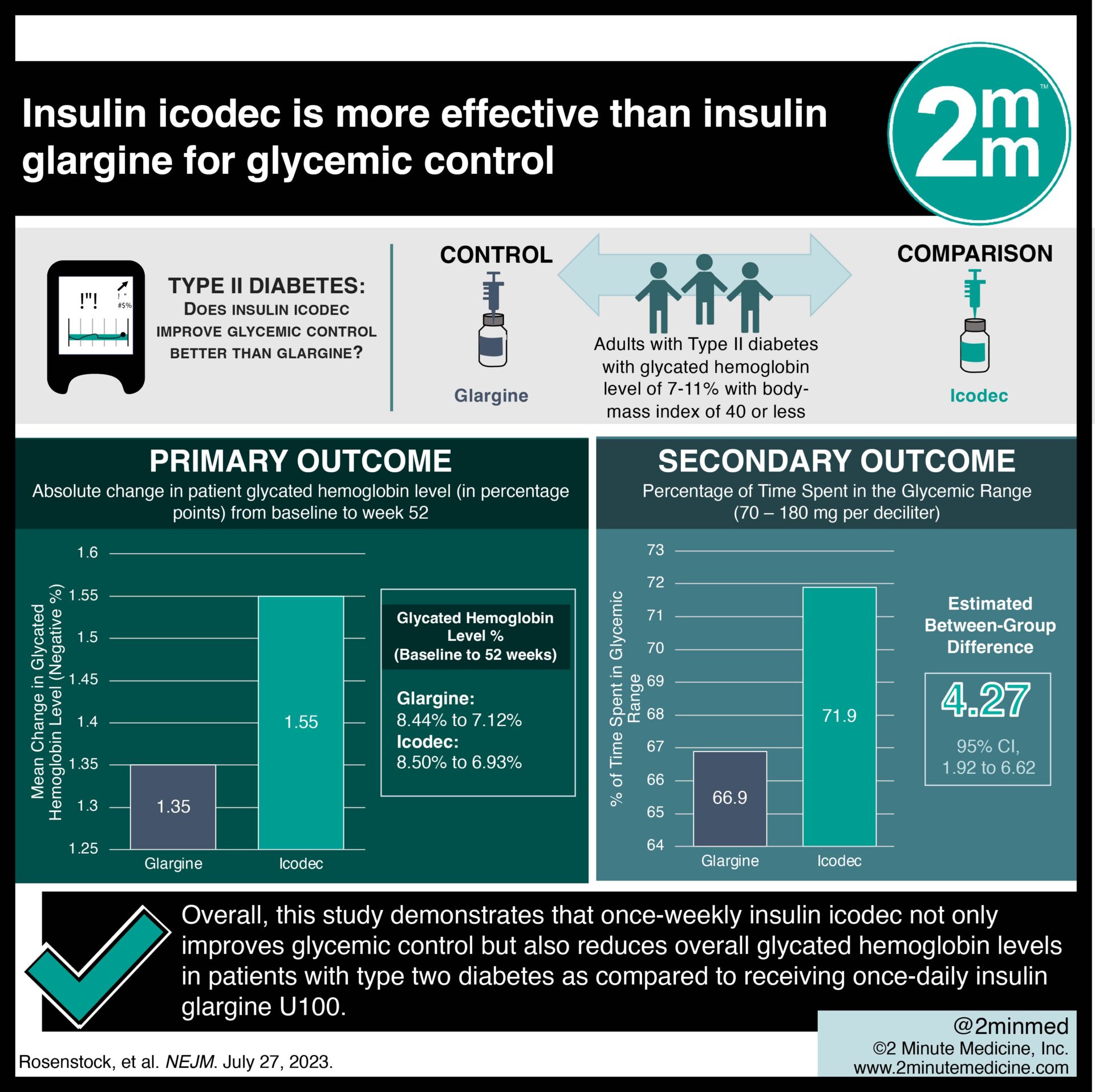
- In this randomized controlled trial, weekly insulin icodec use resulted in better glycemic control than daily insulin glargine in patients with type two diabetes.
- Once-weekly insulin icodec also resulted in a greater reduction in glycated hemoglobin levels than once-daily insulin glargine.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Current treatment for type two diabetes includes initiation of once or twice daily basal insulin analogs to aid proper glycemic control. However, there are concerns regarding adherence to daily injections. It has been demonstrated that once-weekly injectable glucagon-like peptide 1 (GLP-1) receptor agonists are preferred by patients and are associated with improved adherence as well as glycemic control. Yet there remains a gap in knowledge as to understanding whether once-weekly insulin is as effective as daily injections for glycemic control. Overall, this study found that insulin icodec does lead to significantly improved glycemic control and increased likelihood of reaching a glycated hemoglobin level below 7% when compared to the use of glargine U100 in patients with type two diabetes. Notably, this study looked specifically at patients who had not previously received insulin. This study was limited by not having a double-blind, double-dummy design. Nevertheless, these study’s findings are significant, as they demonstrate that once-weekly insulin icodec may be more effective than once-daily insulin glargine in improving glycemic control and reducing overall glycated hemoglobin levels in patients with type two diabetes.
Click to read the study in NEJM
Relevant Reading: Building a Better Insulin — Whom Will It Help?
In-Depth [randomized controlled trial]: This randomized, open-label, treat-to-target, phase 3a trial was conducted in 12 countries across 78 weeks. Patients who were adults (≥18 years of age) with type two diabetes who had a glycated hemoglobin level of 7 to 11% (53.0 to 96.7 mmol per mole) with a body-mass index (the weight in kilograms divided by the square of the height in meters) of 40 or less at screening were eligible for the study. Patients who had previously been prescribed insulin were excluded. The primary outcome measured was an absolute change in patient glycated hemoglobin level (in percentage points) from baseline to week 52. Outcomes in the primary analysis were assessed via hierarchical confirmatory testing. Based on the primary analysis, the mean reduction in the glycated hemoglobin level at 52 weeks was greater with icodec than with glargine U100 (from 8.50% to 6.93% with icodec, mean change, −1.55% and from 8.44% to 7.12% with glargine U100, mean change, −1.35%). Additionally, the noninferiority and superiority of icodec were confirmed by the estimated between-group difference (−0.19%; 95% confidence interval [CI], −0.36 to −0.03). The percentage of time spent in the glycemic range of 70 to 180 mg per deciliter was significantly higher with icodec than with glargine U100 (71.9% vs. 66.9%; estimated between-group difference, 4.27 percentage points; 95% CI, 1.92 to 6.62). In summary, this study demonstrates that once-weekly insulin icodec not only improves glycemic control but also reduces overall glycated hemoglobin levels in patients with type two diabetes as compared to receiving once-daily insulin glargine U100.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.